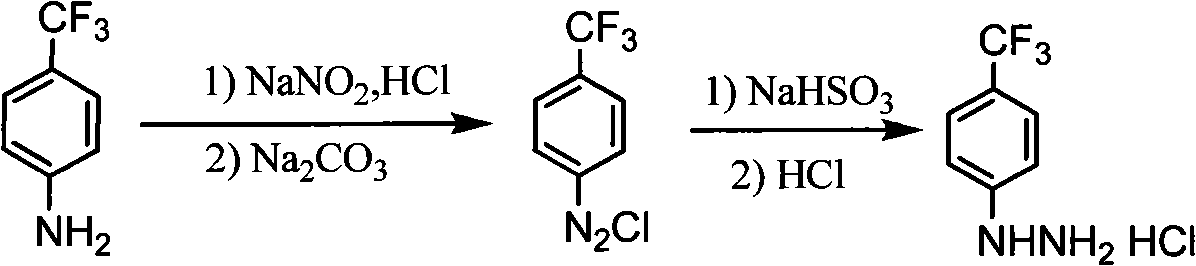
Synthesis method of para-trifluoromethyl phenyl hydrazine hydrochloride
A technology of trifluoromethylphenylhydrazine and trifluoromethylaniline, which is applied in the field of reduction chemical reaction and diazotization, and can solve the problem of poor control of the input amount, inconvenient and troublesome operation, toxicity of stannous chloride Large and other problems, to achieve the effect of low production cost, easy to scale up, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1. Preparation of diazo compound:
[0027] (1) Add 36ml of concentrated hydrochloric acid and 12ml of water into a four-necked bottle with a mechanical stirrer, a thermometer, and an addition funnel, and start stirring.
[0028] (2) p-Trifluoromethylaniline (8.0 g, 0.05 mol) was added dropwise into the reaction flask to form a large amount of white solid, exothermic, and continued stirring to form a paste, the temperature was controlled at 0-10°C.
[0029] (3) Cool the reaction solution to -5°C, add dropwise sodium nitrite solution (prepared from 10.3 grams of sodium nitrite and 20.1 grams of water), and control the temperature at -5 to 0°C. Stir at ~0°C for 2h.
[0030] (4) Add 10 wt% sodium carbonate solution dropwise to adjust the pH value of the diazotization reaction solution to 6-7.
[0031] 2. Reduction reaction:
[0032] (1) Add 125 milliliters of the prepared 25% sodium sulfite solution into a four-necked bottle with a mechanical stirrer, a thermometer and a...
Embodiment 2
[0037] 1. Preparation of diazo compound:
[0038] (1) Add 29ml of concentrated hydrochloric acid and 6.5ml of water into a four-necked bottle with a mechanical stirrer, a thermometer, and an addition funnel, and start stirring.
[0039] (2) p-Trifluoromethylaniline (8.0 g, 0.05 mol) was added dropwise into the reaction flask to generate a large amount of white solid, exothermic, and continued stirring to form a paste with the temperature controlled at 5-10°C.
[0040] (3) Cool the reaction solution to 0°C, add sodium nitrite solution (6.9 grams of sodium nitrite and 12.2 grams of water) dropwise, and control the temperature at 0-5°C. Stir for 1.5h.
[0041] (4) Add 12wt% sodium carbonate solution dropwise to adjust the pH value of the diazotization reaction solution to 5-6.
[0042] 2. Reduction reaction:
[0043] (1) Add 115 ml of prepared 22% sodium sulfite solution into a four-necked bottle with a mechanical stirrer, a thermometer, and an addition funnel, start stirring,...
Embodiment 3
[0048] 1. Preparation of diazo compound:
[0049] (1) Add 15ml of concentrated hydrochloric acid and 5.6ml of water into a four-necked bottle with a mechanical stirrer, a thermometer, and an addition funnel, and start stirring.
[0050] (2) p-Trifluoromethylaniline (8.2 g, 0.051 mol) was added dropwise into the reaction flask to form a large amount of white solid, exothermic, and continued stirring to form a paste, the temperature was controlled at 10-15°C.
[0051] (3) Cool the reaction solution to 5°C, add sodium nitrite solution (5.27 grams of sodium nitrite and 12.3 grams of water) dropwise, and control the temperature at 5-10°C. Stir for 1h.
[0052] (4) Add 11wt% sodium carbonate solution dropwise to adjust the pH value of the diazotization reaction solution to 5-6.
[0053] 2. Reduction reaction:
[0054] (1) Add 77 milliliters of the prepared 25% sodium sulfite solution into a four-necked bottle with a mechanical stirrer, a thermometer and an addition funnel, start ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com