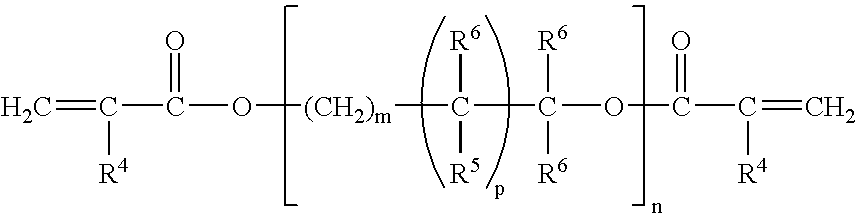
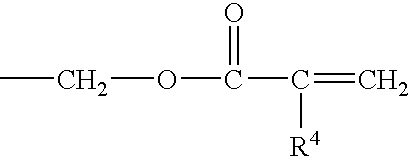
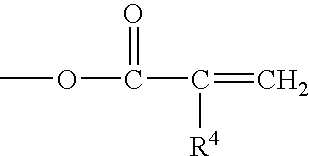
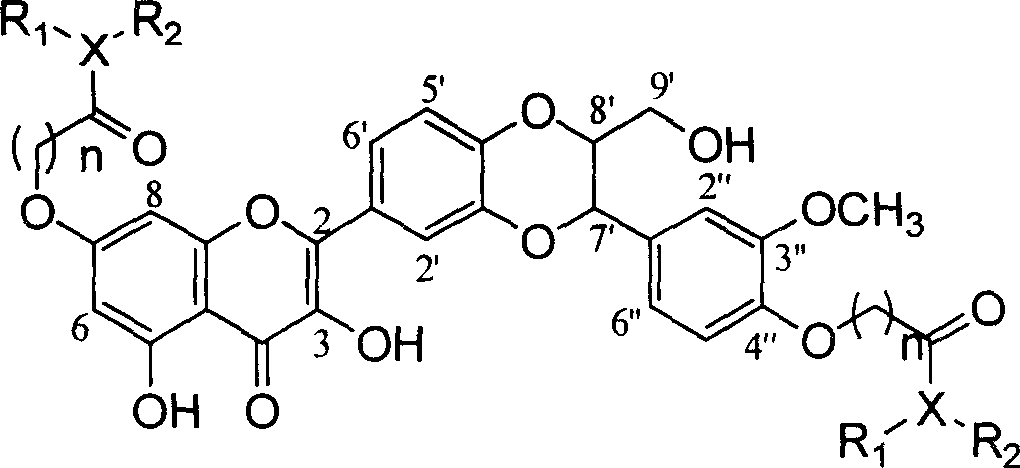
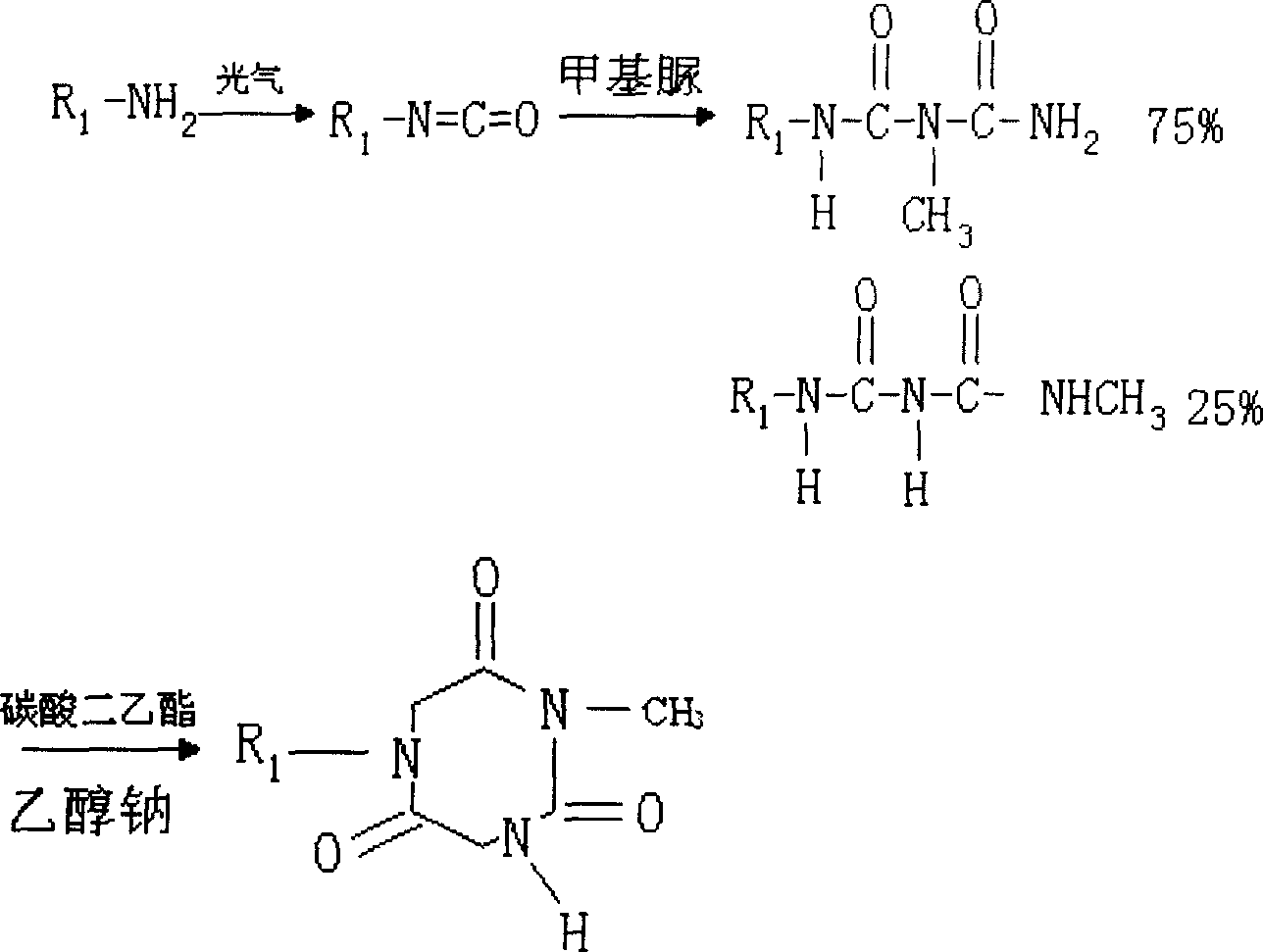
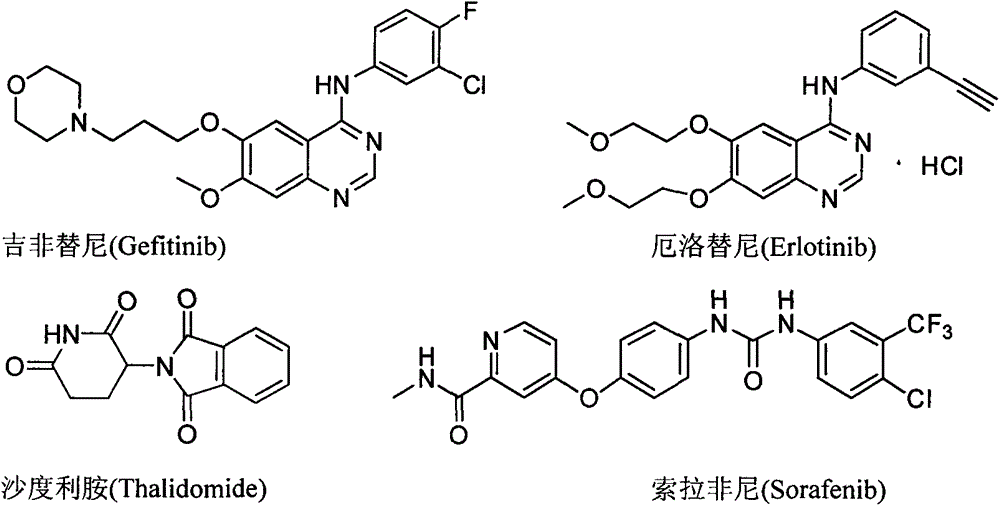
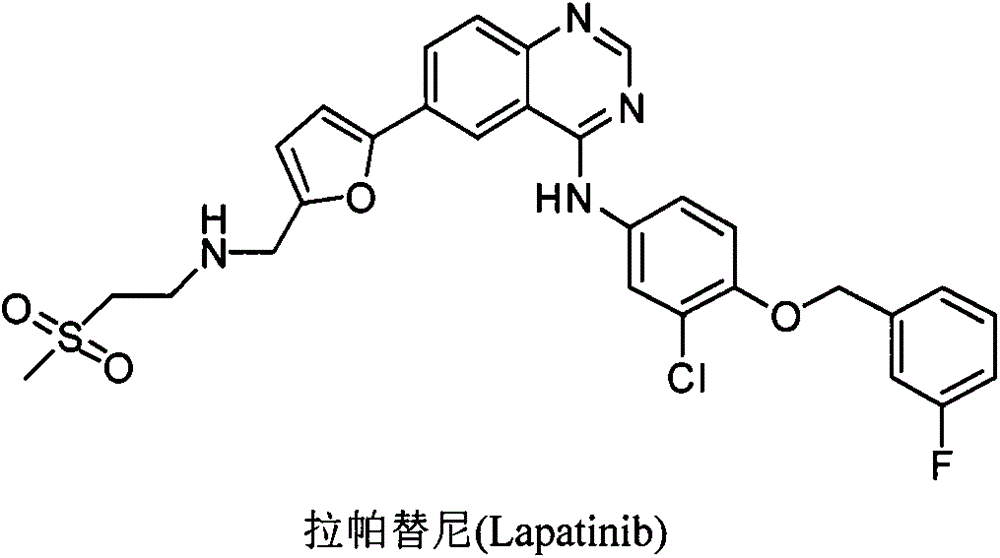
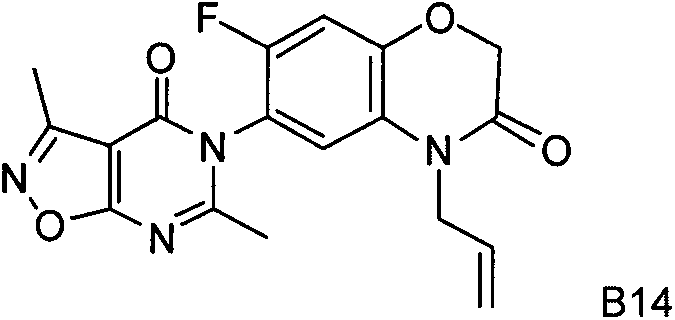
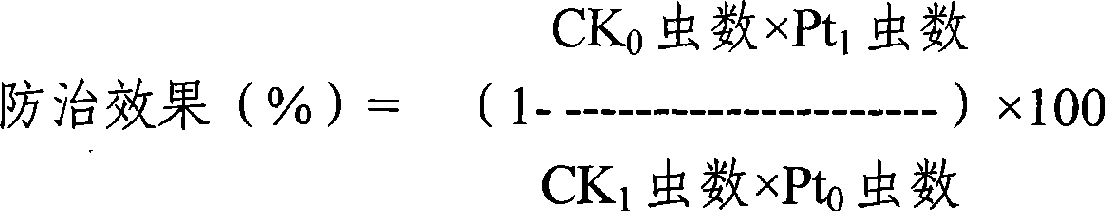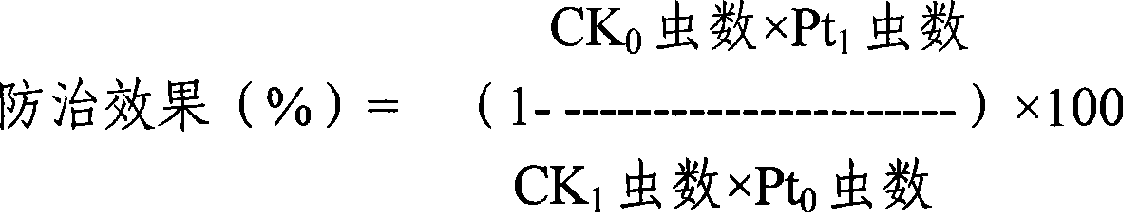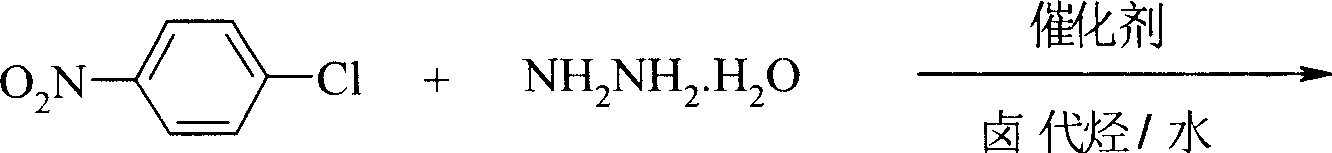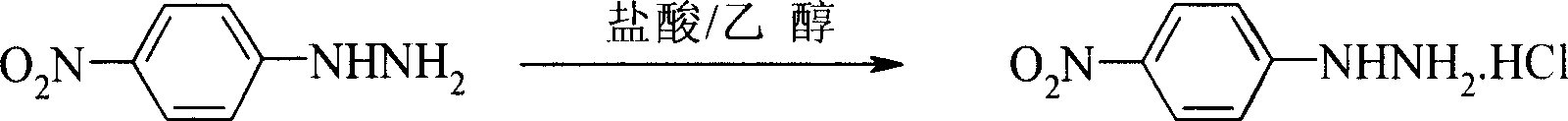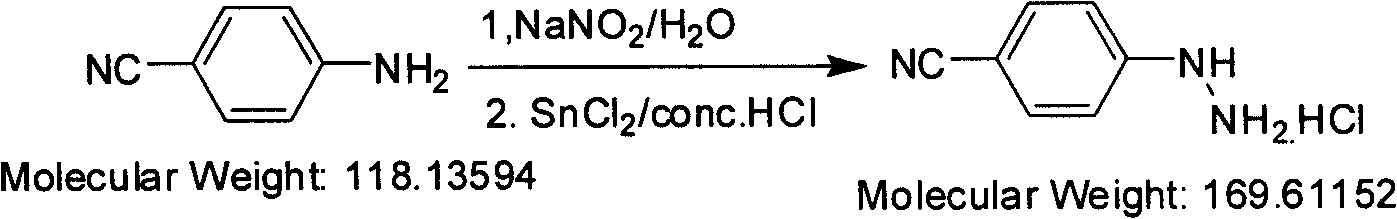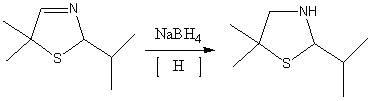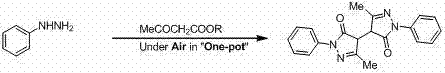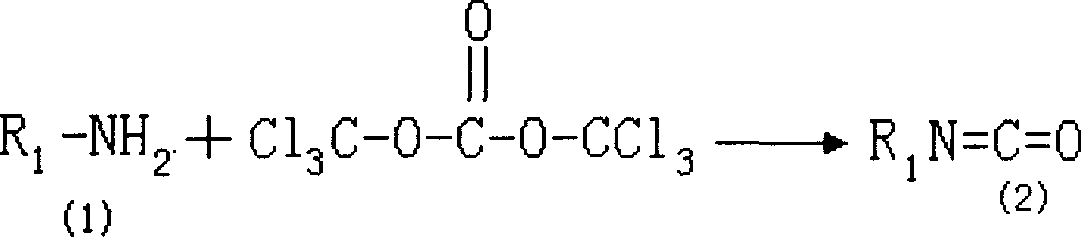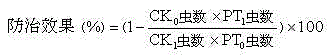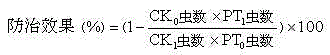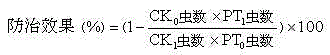Patents
Literature
78 results about "Phenyl hydrazine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Adhesive compositions for bonding passive substrates
InactiveUS20030217808A1Polyureas/polyurethane adhesivesSynthetic resin layered productsHydrazine compoundOnium
Adhesive compositions are disclosed which cure rapidly and completely on confinement between passive substrates, such as magnesium alloys, that are deficient in transition metals and transition metal ions. The compositions include one or more acrylate resins, one or more peroxy free radical initiators, one or more onium salts, and an accelerator such as acetylphenyl hydrazine desirably in an amount of about 1.0% or less by weight of the composition. These compositions provide exceptional bonding to such substrates without the need for a transition metal primer. Methods of making and using such compositions are also disclosed.
Owner:HENKEL IP & HOLDING GMBH
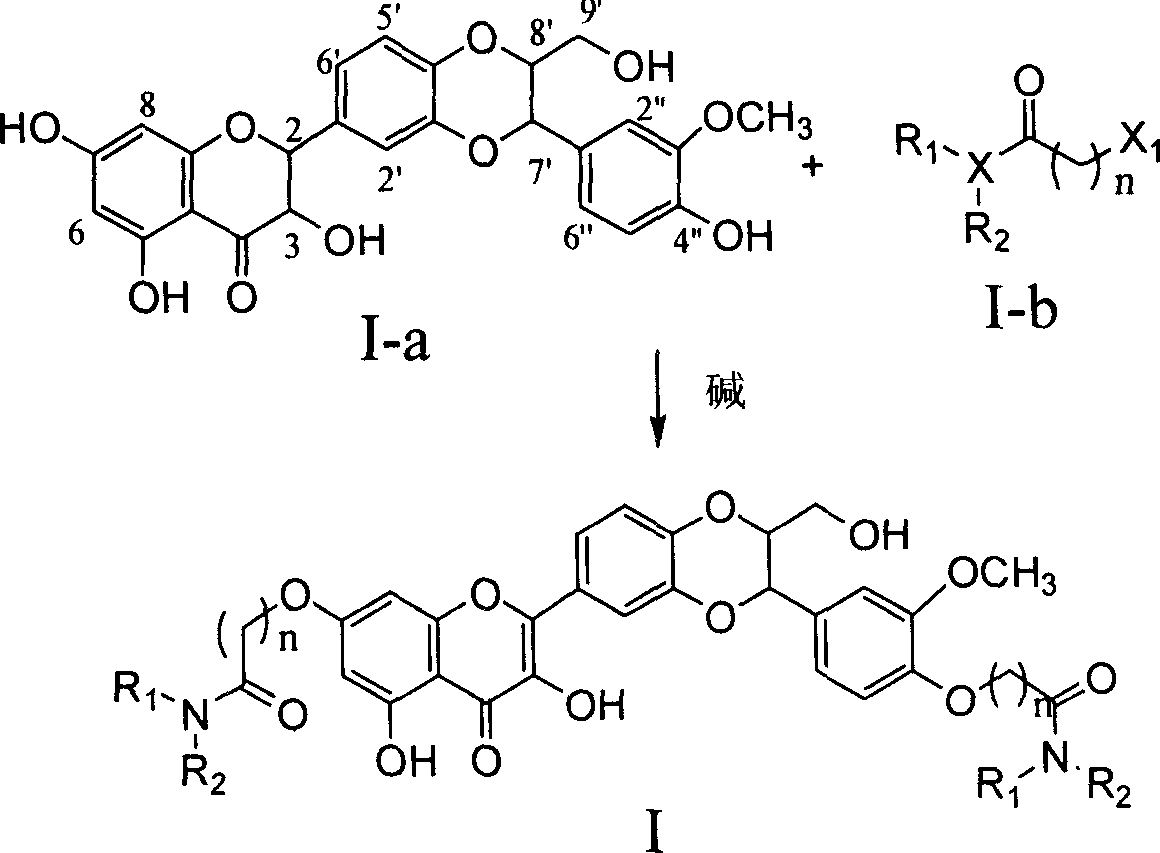
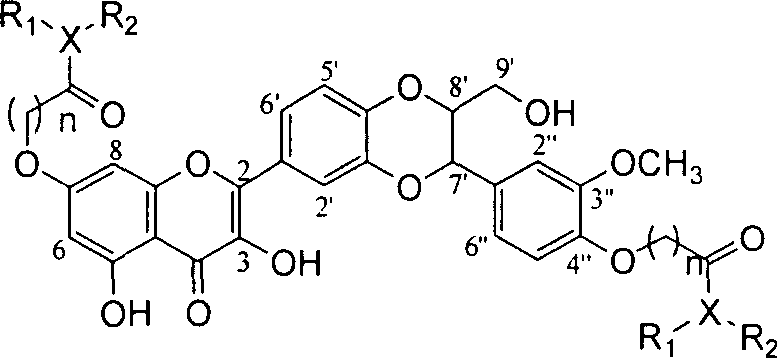
Dehydrosilibinin diester derivatives, preparation method and use thereof
InactiveCN101104616AAntioxidantFree radical scavenging activityOrganic active ingredientsNervous disorderSuperoxideHydrazine compound
The present invention relates to an anti-free radical oxidation, liver-protective, senile dementia preventing and anti-aging active dehydrogenation silybin double-ester derivative and the related medicinal salt or solvent compound. The invention also relates to the preparation method for the compound on the formula 1, as well as the related medication compounds and the curatorial uses. The compound of the invention can protect the liver cells of a rat liver cell injury in vitro model, which can be expected to prevent liver damage in drug use; the compound of the invention is provided with the biological activities of in vitro removing superoxide anion free radicals and diphenyl-benzyl-hydrazine free radicals, and restraining generation of grease peroxide induced by free radicals. The compound can strongly confront the PC12 cells damage caused by free radicals, which can be expected to prevent various diseases caused by free radicals in drug use.
Owner:ZHEJIANG UNIV
Bifenazate-containing insecticidal composition
InactiveCN101530109AImprove securityMeet security requirementsBiocideAnimal repellantsActive componentSynergy
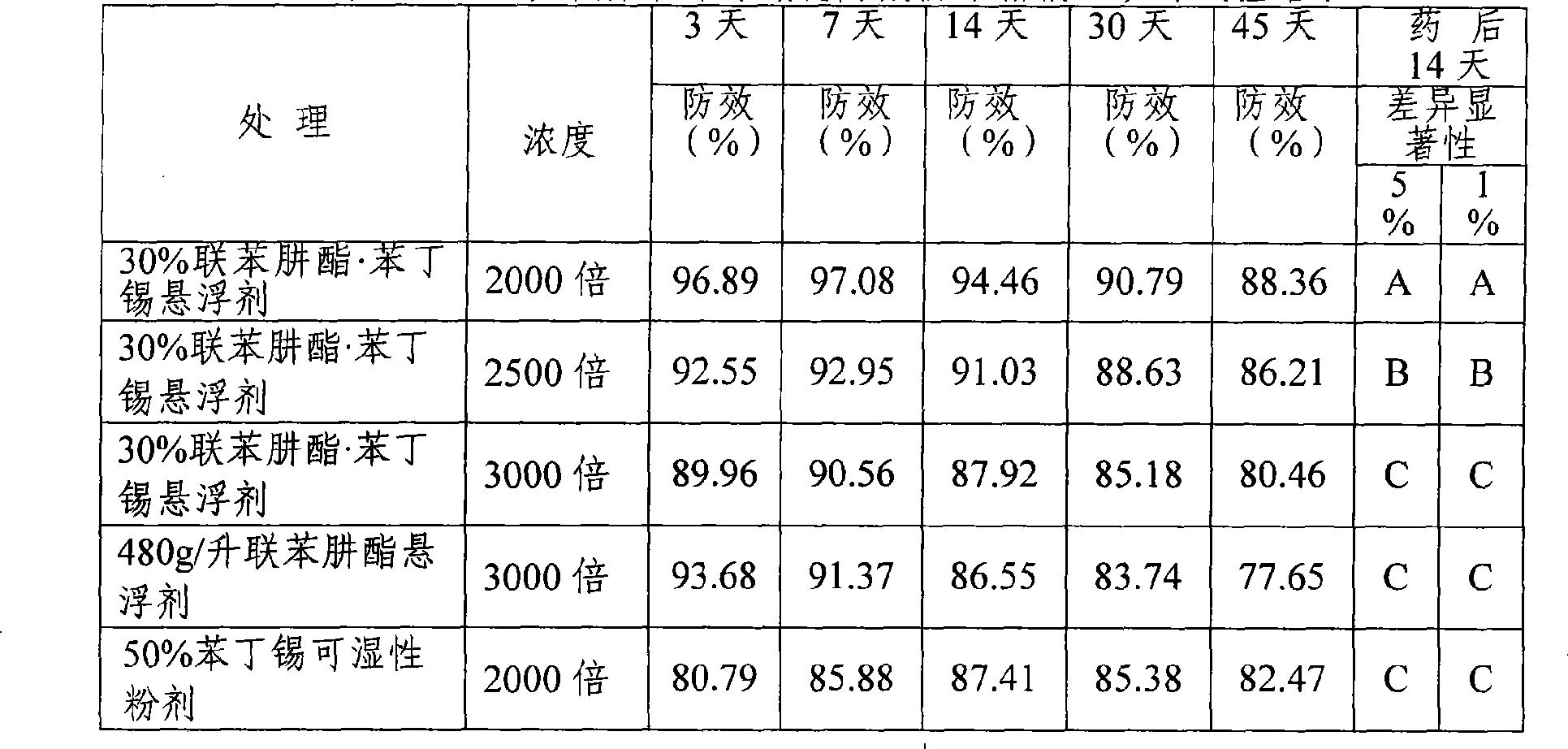
The invention discloses a bifenazate-containing agricultural insecticidal composition and application thereof. The composition contains bifenazate as a first active component and contains fenbutatin oxide or clofentezine as a second active component, wherein the weight ratio of the first active component to the second active component is 0.01-200:1, preferably 0.2-20:1. The composition has the advantages that the composition is reasonable in components, good in insecticidal effects and low in drug-using cost; in addition, the activity and insecticidal effects of the composition have remarkable synergy instead of simply adding the activity of each component, and the composition is quick in response and long in lasting effects, slows down resistance generation, is good in safety to crops, and meets the requirement of pesticide preparations on safety. The prevention and control effect of the composition on citrus red mites, European red mites and mites on cotton crops reaches 86.65 to 96.38 percent.
Owner:SHAANXI SUNGER ROAD BIO SCI
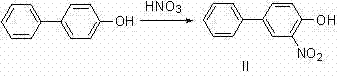
Synthesis method for bifenazate
The invention belongs to the technical field of pesticide and midbody synthesis, particularly relates to a synthesis method for bifenazate. In the synthesis method for the bifenazate, a target product is synthetized by taking para-hydroxydiphenyl as a raw material through nitration, methylation, hydrogenated reduction, diazotization, diazonium salt reduction and an acylation one-step method, wherein acylation reaction is directly performed on hydrazonium salt without treatment, thus, the experimental procedure is shortened, the yield is improved to above 65 percent from 50 percent, the conversion rate of a reaction product is high, and the purification treatment is easy to perform. The synthesis method for the bifenazate has the advantages that the operation is simple, the condition is mild, the yield is high, the cost is low, and the like, is easy to carry out industrial production and has a wide application prospect. The bifenazate synthetized by the invention can be used as a specific acaricide, is active in active stage of tetranychid, can also control pests, such as Tetranychus urticae, in the egg stage, and is used for cotton, hop and certain fruiter crops to effectively prevent all mites in the growth stage.
Owner:TONGJI UNIV
Acaricide compound containing biphenyl hydrazine ester
ActiveCN101697728AImprove securityGood control effectBiocideAnimal repellantsPropargiteHydrazine compound
The invention relates to an acaricide compound containing biphenyl hydrazine ester. The weight precentage ratio of the first active ingredient: biphenyl hydrazine ester and the second active ingredient: abamectin, pyridaben or propargite of the compound is (5 to 40): 1, (0.5 to 8):1, (0.125 to 0.5):1. The acaricide compound can be prepared into water dispersed particle agent, suspending agent, wettable powder, missible oil or aqueous emulsion. The compound can be applied to the citrus red mite, European red mite and mite on cotton plants. The component is reasonable, the mite killing effect is obvious and the manufacturing cost is low. With quick acting property and long lasting effect, the activity and the disinfectant effect of compound are obviously beneficiated, can slow down the generation of resistance, are safe to plant and meet the safety demand of agricultural chemical preparation.
Owner:SHAANXI SUNGER ROAD BIO SCI
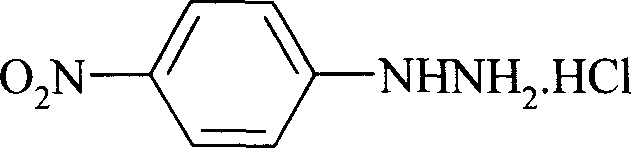
Preparation process of p-nitro phenyl hydrazine hydrochloride
InactiveCN100999483AMild reaction conditionsSimple and safe operationHydrazine preparationHydrazine compoundNitrobenzene
The invention relates to a p-Nitrophenylhydrazine muriate preparation methods. In halogenated hydrocarbonand water two-phase system, take p-cl-nitrobenzene as raw material to take reaction with hydrazine hydrate under catalyst, obtain p-Nitrophenylhydrazine, then through hydrochloride to obtain product of the invention. With this method, the purity of p-Nitrophenylhydrazine muriate can achieve more than 99%, 80 to 85% yield.
Owner:SHANGHAI CHEM REAGENT RES INST
Preparation method and application of pH sensitive drug delivery system with carbon nanomaterial as carrier
InactiveCN103191440AEnergy modified materialsPharmaceutical non-active ingredientsTumor therapyPharmaceutical Substances
The invention relates to a preparation method and application of a pH sensitive drug delivery system with a carbon nanomaterial as a carrier. The pH sensitive drug delivery system can be used for effectively solving the problems that the traditional tumor therapy drugs are high in side effect and incapable of well inhibiting the tumor cell proliferation. The technical scheme for solving the problems is as follows: the preparation method comprises the following steps of: modifying the original carbon nanomaterial, and then, subjecting the modified original carbon nanomaterial and an ammoniation reagent to a reaction; after connecting an amino with reaction activity to the surface of the carbon nanomaterial, subjecting the carbon nanomaterial and p-hydrazinobenzoic acid to a reaction to generate a p-hydrazinobenzoic acid derivative of the carbon nanomaterial; and then, subjecting the p-hydrazinobenzoic acid derivative and a carbonyl contained anti-tumor drug to a reaction to obtain the pH sensitive drug delivery system with the carbon nanomaterial as the carrier, wherein the carbon nanomaterial is one selected from fullerene, a carbon nanotube and graphene oxide. The pH sensitive drug delivery system is good in biocompatibility, large in specific surface area and high in chemical inertness, has a control release feature and a targeting property, and is an innovation of the anti-tumor drug.
Owner:ZHENGZHOU UNIV
Preparation method of paracyano-group phenyl hydrazine hydrochloride
InactiveCN101550091ALower requirementReasonable choice of preparation processHydrazine preparationHydrazine compoundAniline
The invention relates to a preparation method for compounding fragrant hydrazine, in particular to a preparation method of paracyano-group phenyl hydrazine hydrochloride, which mainly solves the technical problems of poor crystal shape and low yield of the prior preparation method. The adopted technical scheme is as follows: the preparation method of the paracyano-group phenyl hydrazine hydrochloride is characterized in that: paracyano-group aniline is used as a raw material and is prepared into diazo hydrochloride in a hydrochloric water solution by the diazotizing reaction of sodium nitrite in the mode of one-step continuous reacting operation, the diazo hydrochloride is reduced by a tin-protochloride hydrochloric solution, filtered and washed to obtain paracyano-group phenyl hydrazine hydrochloride, and the paracyano-group phenyl hydrazine hydrochloride is recrystallized by the mixed solution of methanol and water to obtain a white crystal target product. The paracyano-group phenyl hydrazine hydrochloride obtained by the invention is an important normal medical intermediate product.
Owner:上海药明康德新药开发有限公司
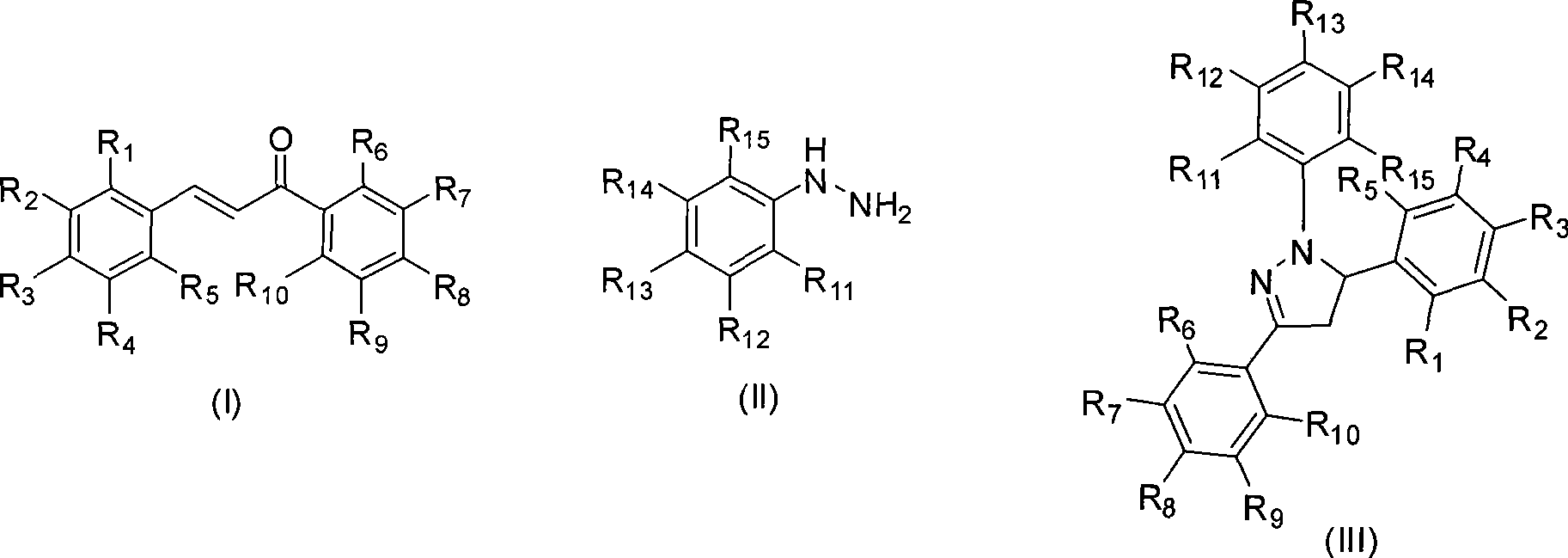
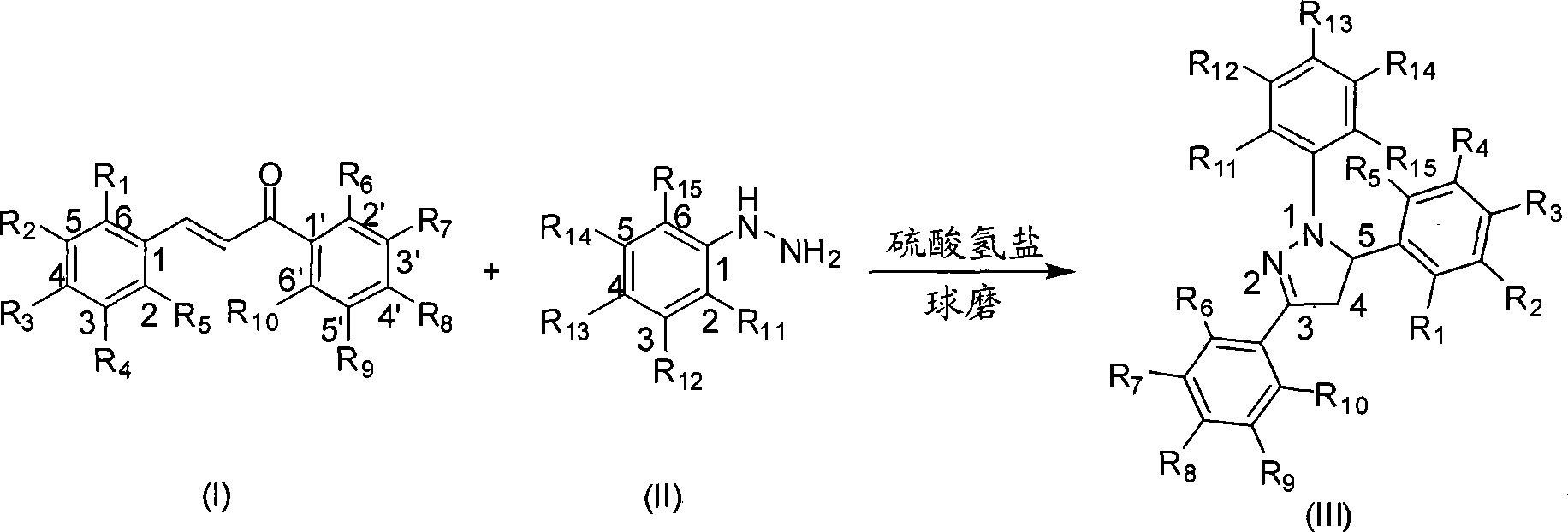
Mechanochemical preparation of 1,3,5-triaryl-2-pyrazoline compounds
InactiveCN101440065AWiden the optionsSolve the problem of large yield variationOrganic chemistrySilica gelRaw material
The invention discloses a mechanochemical preparation method for a 1, 3, 5-triaryl-2-pyrazoline compound with a structure shown in a formula (III). The method comprises the following steps: a chalcone compound with a structure shown in a formula (I) and a phenyl hydrazine compound with a structure shown in a formula (II) are taken as raw materials, hydrosulfate is taken as a catalyst, and silica gel is taken as a grinding aid to perform a ball milling reaction in a closed ball milling tank; and after the reaction is finished, a reaction mixture is separated and purified to obtain the 1, 3, 5-triaryl-2-pyrazoline compound with the structure shown in the formula (III). The method has the advantages of safe and reliable production, simple and convenient operation, short reaction time, generally higher reaction yield, low production cost, simple post-treatment, small pollution and so on, and is the preparation method for the 1, 3, 5-triaryl-2-pyrazoline compound with better popularization and application prospects.
Owner:ZHEJIANG UNIV OF TECH
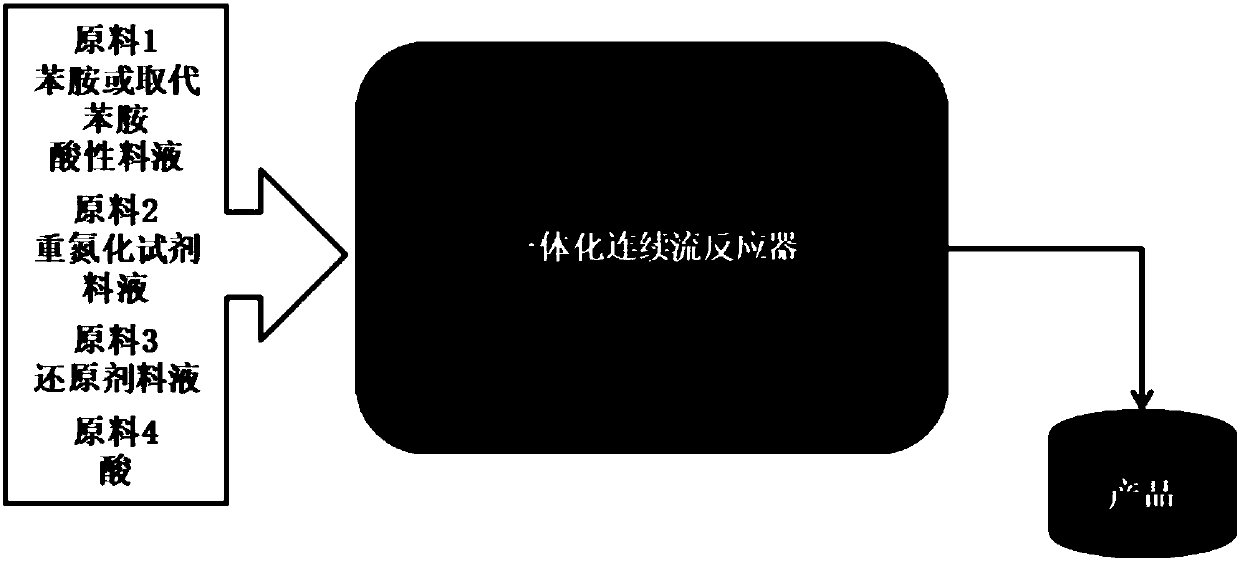
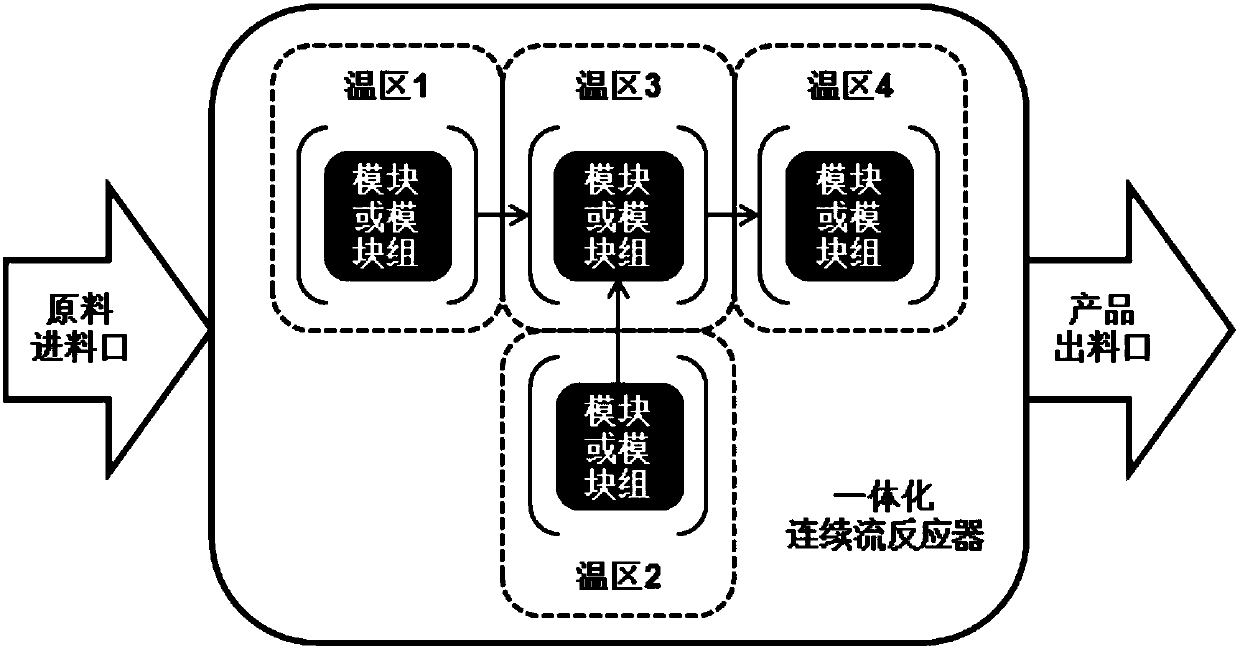
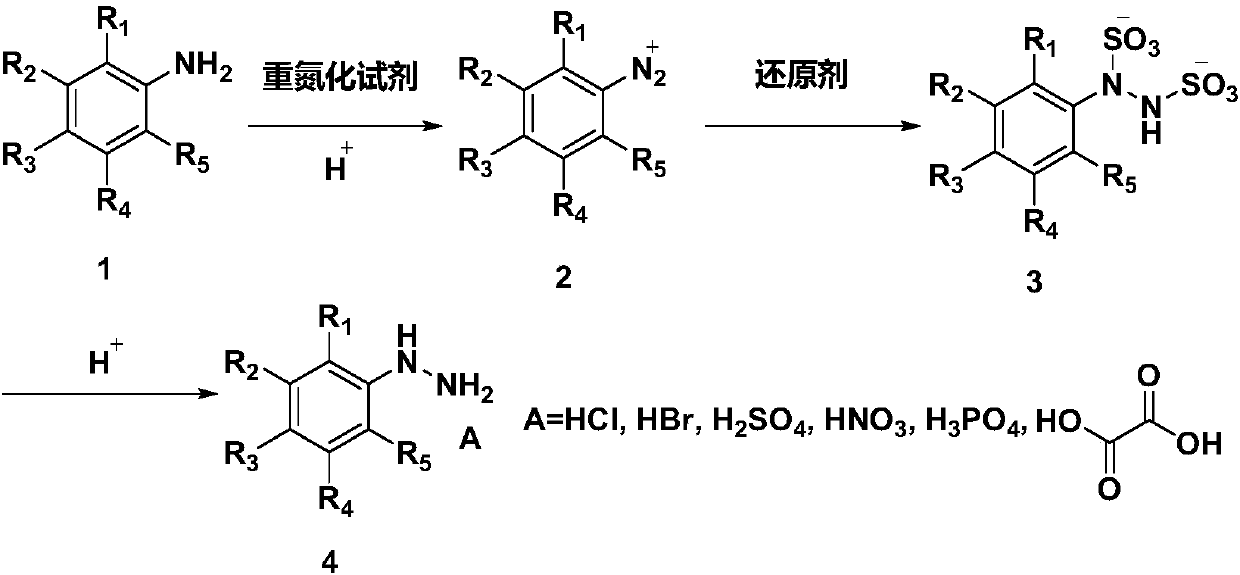
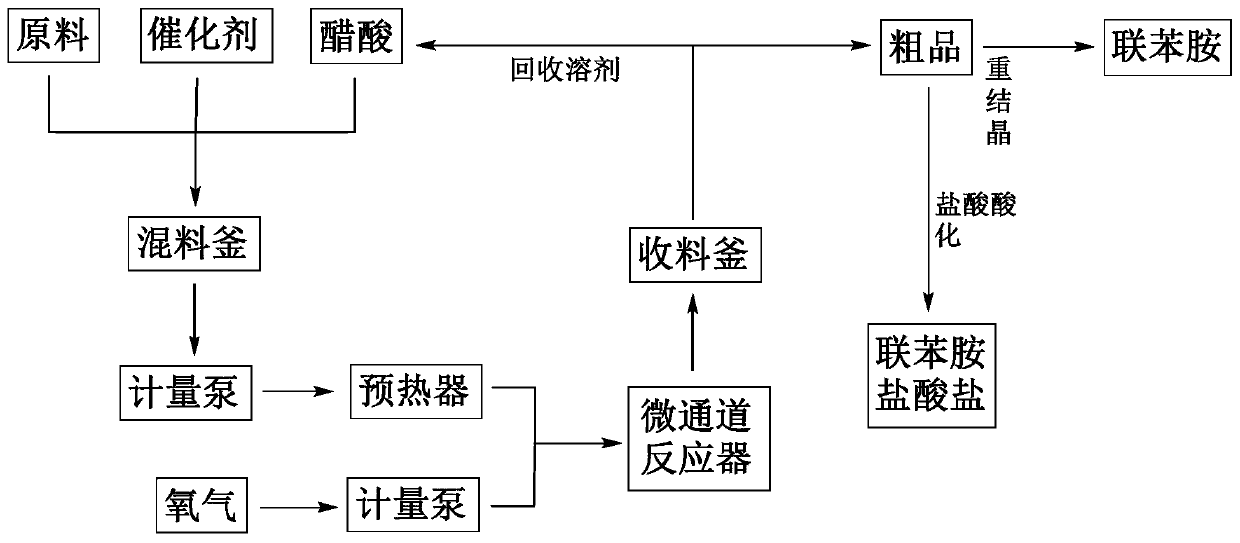
Phenyl hydrazine and substituted phenyl hydrazine continuous flow synthesis process
ActiveCN107663161ARealize the whole process of continuous flow synthesisSolve the technical problems of continuous production in the whole processHydrazine preparationSequential/parallel process reactionsReaction intermediateDiazoamino Compounds
The invention provides a phenyl hydrazine and substituted phenyl hydrazine continuous flow synthesis process. Three-step reactions of diazotization, reduction and acidolysis salt formation are creatively and organically integrated; phenylamine or substituted phenylamine acid material liquid, diazotization reagents, reducing agents and acid are used as raw materials to be subjected to three-step reaction of diazotization, reduction and acidolysis salt formation to obtain phenyl hydrazine derivative salts. The synthesis process is performed in an integral reactor, and belongs to an integrated solution. The reaction raw materials are continuously added through a feeding opening of the integral reactor; the reactions of diazotization, reduction and acidolysis salt formation are continuously performed in the integral reactor; the phenyl hydrazine derivative salts are continuously obtained in the integral reactor; the total reaction time is less than or equal to 20min. Compared with a conventional production process, the continuous flow synthesis process has the advantages that the total reaction time is greatly shortened; the safety is greatly improved; no reaction byproducts (such as diazoamino compounds and reduction reaction midbodies) are included in the reaction process and the reaction products; the continuous flow process can prepare the high-purity products with the purity as high as 99 percent or higher without the additional purification steps.
Owner:SHANGHAI HYBRID CHEM TECH
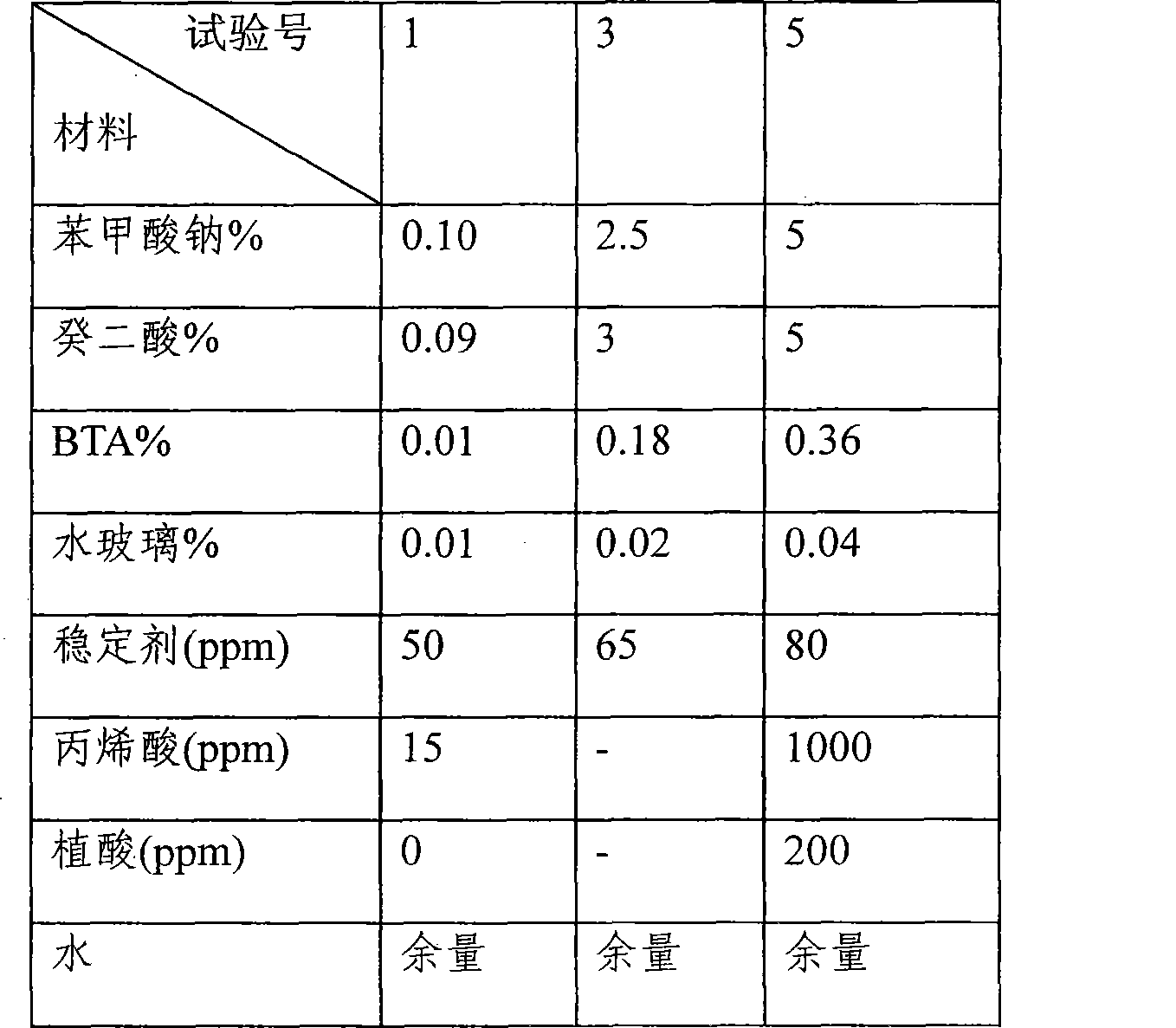
Corrosion inhibitor of internal combustion engine cooling liquid and production process
InactiveCN101381170AInhibit heat transfer corrosionInhibition of Vibration Cavitation CorrosionScale removal and water softeningOrganic acidChemistry
The present invention discloses a novel engine coolant inhibitor, which belongs to the novel inhibitor of the internal combustion engine circulating cooling media. The complex production process of the inhibitor is a special method for preparing a complex inhibitor. The inhibitor is prepared from a formulation that unitary organic acid, binary organic acid, hydroxyphenyl hydrazine, phytic acid, siliceous compounds and siloxane stabilizer thereof, azole compounds, water stabilizer are added with pure water according to a weight ratio thereof. The inhibitor adopts a production process that the chemical agents are divided into class A and class B, which are respectively measured and packed after respectively mixed and mechanically and evenly grinded. The inhibitor has the characteristics of easy and quick dissolution, convenient use, convenient storing, convenient transportation, simple measurement, low price, and the like. The inhibitor is a novel inhibitor which is environment friendly, highly efficient, and good in corrosion inhibition performance.
Owner:袁晓东 +2
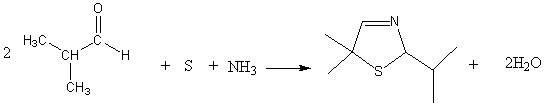
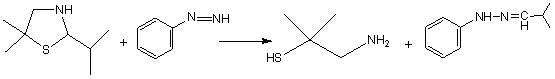
Synthetic method of dimethyl cysteamine hydrochloride
ActiveCN102432510AHigh purityReaction raw materials are readily availableThiol preparationThiazolineCYSTEAMINE HYDROCHLORIDE
The invention discloses a synthetic method of dimethyl cysteamine hydrochloride. The method comprises the following steps of: synthesizing 5,5-dimethyl-2-isopropyl thiazoline by taking isobutylaldehyde, element sulfur, ammonia gas and triethylamine as raw materials; reducing the 5,5-dimethyl-2-isopropyl thiazoline into 5,5-dimethyl-2-isopropyl thiazolidine under the actions of sodium borohydride and acid; and reacting the 5,5-dimethyl-2-isopropyl thiazolidine and phenyl hydrazine under the air insulating situation to obtain dimethyl cysteamine hydrochloride. The method has the advantages of readily-available reaction raw materials, easiness for operating the reaction process, low requirement on the reaction equipment, relatively mild reaction conditions, feeding of the product of every step of reaction into a next step reaction after refining and purifying, high yield and high purity, and the purity of the finally-obtained dimethyl cysteamine hydrochloride is close to 100 percent.
Owner:山东胜利生物工程有限公司
Organic one-component inserted bar glue and preparation method thereof
InactiveCN104762052AFor long-term storageLow viscosityNon-macromolecular adhesive additivesModified epoxy resin adhesivesPolymer scienceAcrylic adhesive
The invention discloses an organic one-component inserted bar glue and a preparation method thereof, and belongs to the technical field of anaerobic adhesives. The organic one-component inserted bar glue is prepared from the following raw materials: methacrylic acid epoxide, triethylene-glycol dimethacrylate, acrylic acid, ethylenediamine tetraacetic acid, cumene hydroperoxide, acidified saccharin and hydracetin. By virtue of an anaerobic curing mechanism, the target of one-component inserted bar glue is reached; and the acrylic adhesive can be relatively permanently stored in a liquid state when oxygen exists, and can be cured into an insoluble and non-fusible solid at a room temperature after being isolated from air.
Owner:DALIAN UNIV OF TECH
Biphenyl hydrazine ester suspension concentrate and preparation method thereof
The invention discloses biphenyl hydrazine ester suspension concentrate, which consists of the following components in percentage by weight: 1%-50% of biphenyl hydrazine ester, 1%-8% of a dispersing agent, 1%-10% of a wetting agent. 0.1%-2% of a thickening stabilizer, 1%-10% of an antifreezing agent, 0.1%-1% of a foaming killer, 0.1%-0.5% of preservative and the balance of water, wherein a pH regulating agent is used for regulating pH value to be 6-8. The invention further discloses a preparation method of the biphenyl hydrazine ester suspension concentrate. The invention provides an environment-friendly biphenyl hydrazine ester water-based suspension concentrate dosage form, which has the advantages of high flash point, safety in production, storage and application, small environmental pollution, low production cost, easiness in packing and the like, and can efficiently and safely meet the requirements of agricultural production.
Owner:成都科利隆生化有限公司
Preparation method for 4-sulfonamidophenylhydrazine hydrochloride
InactiveCN101148430AShort reaction timeHigh puritySulfonic acid amide preparationSodium metabisulfiteAniline
The present invention relates to preparation process of p-sulfonylamino phenyl hydrazine. The compound p-sulfonylamino phenyl hydrazine is prepared with p-sulfonylamino aniline as initial material, and through diazo reaction, reduction reaction and hydrolysis. The reduction reaction has sodium pyrosulfite as the reductant and the reaction conditions of 10-35 deg.c temperature and pH 7-9. The p-sulfonylamino phenyl hydrazine preparing process has high product purity and low production cost.
Owner:太仓市华联化工实业有限公司
Method for preparing diphenyl hydrazine
InactiveCN101838219ARaw materials are easy to getLow costHydrazine preparationHydrogen pressureHydrazine compound
The invention relates to a method for preparing diphenyl hydrazine. The method comprises the steps of: adding nitrobenzene, absolute methanol, sodium hydroxide and a certain mass of Raney nickel in a stainless steel reactor; introducing 0.05-0.10 MPa of ammonia gas; maintaining the pressure of hydrogen at 0.2-0.8 MPa; releasing the pressure after reaction for a period by stirring at a room temperature; pouring a reaction liquid out; stirring and soaking the catalyst at the bottom of the reactor with methanol for one time; blending the catalyst soaking liquid, i.e., methanol, with the reaction liquid, and then pouring into a separating funnel; adding a proper amount of diluted HCl for neutralization; separating the water layer at the lower part out after being neutral; extracting with methanol; pouring the blended liquid of a grease layer and an extraction liquid into a flask; and after obtaining a part of solvent methanol by decompression and distilling, separating out yellowy plate crystals, i.e., diphenyl hydrazine, by cooling and crystallization. The method has the characteristics of high yield, easy operation and the like.
Owner:周成云
Novel preparation method and use of bisedaravone and medicinal salts of bisedaravone
InactiveCN102432540AHigh yieldImprove solubilityOrganic chemistryAntinoxious agentsAcetoacetatesFree radicals scavenger
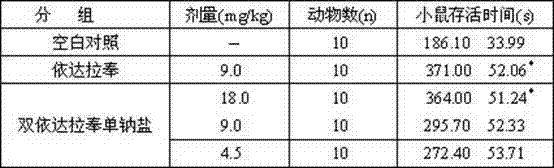
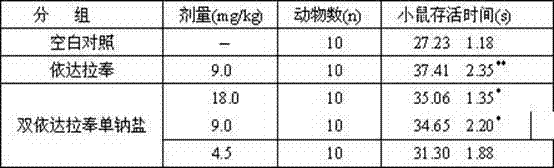
The invention belongs to the technical field of medicine synthesis and relates to a novel preparation method and a use of bisedaravone and medicinal salts of bisedaravone. The preparation method comprises the fo0llowing steps that hydrazinobenzene and acetoacetic ester undergo a reaction in the air or oxygen atmosphere to produce edaravone; and the obtained edaravone undergoes an oxidation dimerization reaction to produce bisedaravone. The novel preparation method has a high yield. Through the novel preparation method, bisedaravone solubility is improved. The medicinal salts of bisedaravone comprise monosodium salts, disodium salts, monopotassium salts, dipotassium salts, monolithium salts and dilithium salts, have free radical scavenger effects, and can be utilized for clinical treatment on cerebral infarction.
Owner:SHENYANG PHARMA UNIVERSITY +1
Production of tolyl-triazone
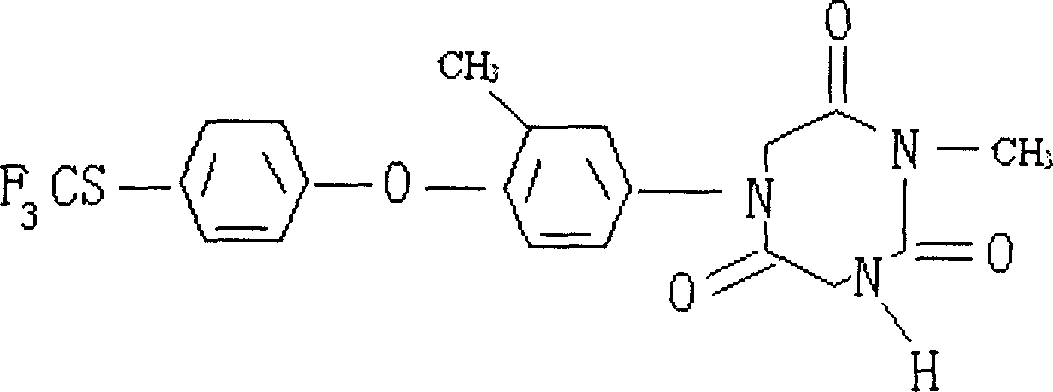
Production of tolyl triazone is carried out by synthesizing 3-methyl-4-(4-methylthio-trifluoride) phenoxy phenyl-hydrazine acid ester, preparing 1(N)-acetyl-2(N)-methyl-3(N)-(3-methyl-4-(4- methylthio-trifluoride)phenoxy) phenyl-triuret, preparing 1(N)-methyl-3(N)-hydrogen-5(N)-(3-methyl-4-(4- methylthio-trifluoride)phenoxy) phenyl-1,3,5-triazine-2,4,6-triketone and purifying crude product. It has better content and purity, more yields and no environmental pollution.
Owner:JIANGSU LINGYUN PHARMA
1-substitution phenyl-4-polysubstitution phenyl-5-methylmercapto-1H pyrazole compound with anti-hepatoma activity
The invention relates to the synthesis and anti-hepatoma activity study of a 1-substitution phenyl-3-methyl-4-(2-fluorine-4-chlorine-5-propargyloxyphenyl)-5-methylmercapto-1H pyrazole compound (A). The compound (A) is prepared through the following steps of reducing 2-chlorine-4-fluorine-5-nitro phenyl ethyl carbonate with iron powder, carrying out diazotization and Meerwein arylation reaction and reacting with carbon disulfide and dimethyl sulfate to obtain 2-chlorine-4-fluorine-5-(1,1-dimethylthio-3-oxo-1-alkene-2-group) phenyl ethyl carbonate (5), hydrolyzing the (5), then reacting with propargyl bromide to obtain 4,4-dimethylthio-3-(2-fluorine-4-chlorine-5-propargyloxyphenyl) butane-3-alkene-2-ketone (6), and finally with ethyl alcohol as a solvent, reacting (6) with different substituted phenylhydrazine hydrochlorides under reflux condition to generate the compound (A). Test results indicate that both the degrees of the compound A for inhibiting HepG2 cancer cells and promoting cell apoptosis are greater than or equal to control drug cyclophosphamide.
Owner:NANKAI UNIV
Preparation process for 4- bromo phenyl hydrazine
InactiveCN102382010ALow priceReduce manufacturing costHydrazine preparationSulfonic acid amide preparationSodium metabisulfiteHydrogen
The invention relates to preparation process for 4- bromo phenyl hydrazine. The method is characterized in that arylamine is adopted as an initial raw material, a 4- bromo phenyl hydrazine product is obtained after diazotization reaction, reduction reaction, purification and drying sequentially, reducing agent of the reduction reaction is sodium metabisulfite, and the reduction reaction is carried out under conditions that temperature ranges from 10 DEG C to 35 DEG C and pH (potential of hydrogen) is 7. A 4- bromo phenyl hydrazine product prepared by the preparation method is excellent, and production cost is low.
Owner:袁雪冲
Bifenazate-containing insecticidal composition
InactiveCN103329913AImprove securityMeet security requirementsBiocideAnimal repellantsToxicologyChemistry
The invention discloses a bifenazate-containing insecticidal composition. Active components comprise bifenazate and clofentezine, wherein the weight ratio of the bifenazate to the clofentezine is 0.01-200:1, preferably 0.2-20:1. The composition is advantageous in that the composition has reasonable components, good insecticidal effects and low drug-using cost; activity and insecticidal effects of the composition are not simply addition of activity of each component but have remarkable synergy effects; the composition has high rapid-acting property and long effect persistence; the composition slows down generation of resistance; and the composition is good to crop safety, and meets safety requirements of pesticide preparations. The control efficiency of the composition for citrus red mites, apple red mites, and mites on cotton crops reaches 86.65%-96.38%.
Owner:SHAANXI SUNGER ROAD BIO SCI
Process of measuring carbonyl compound content in oil and grease
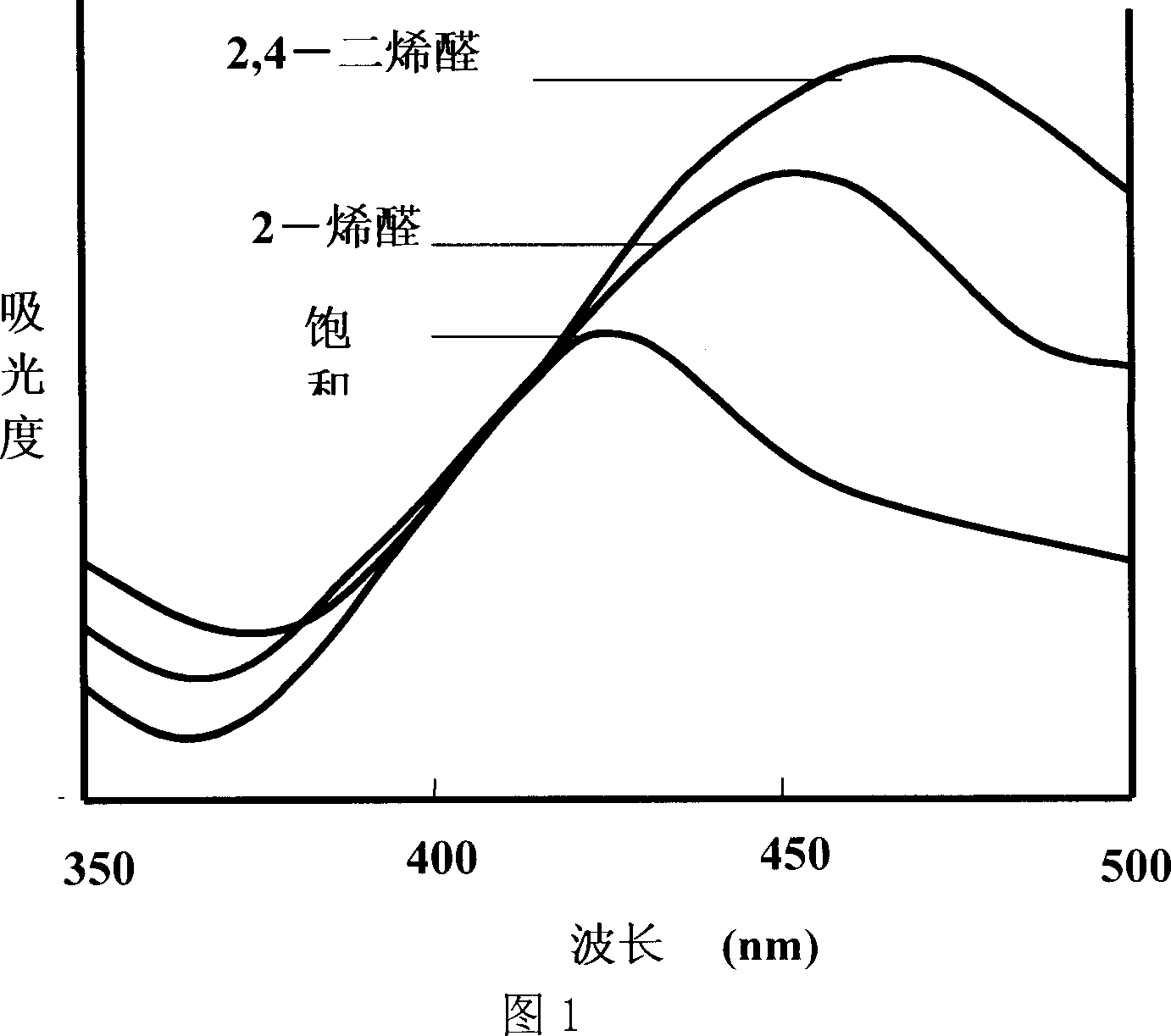
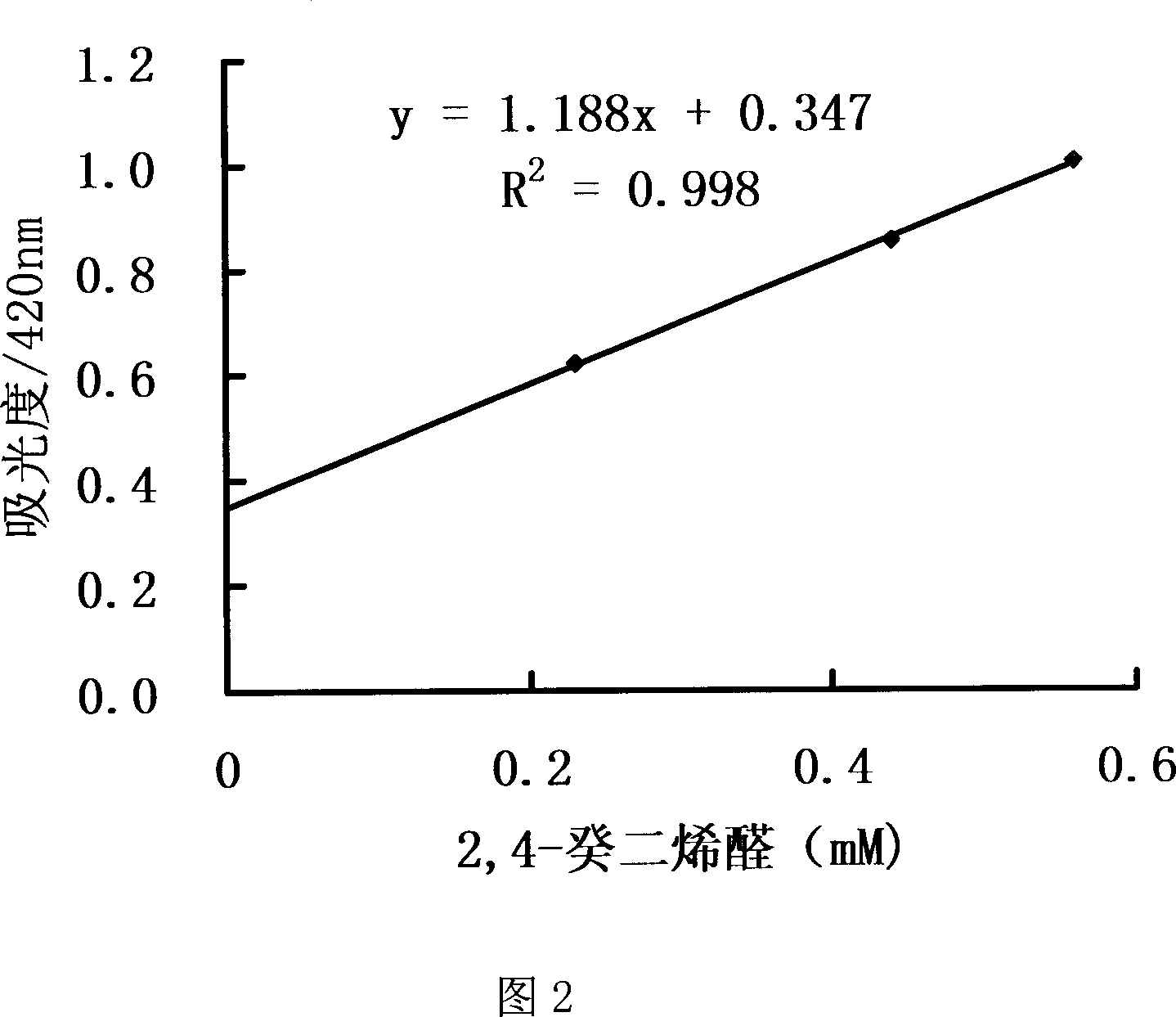
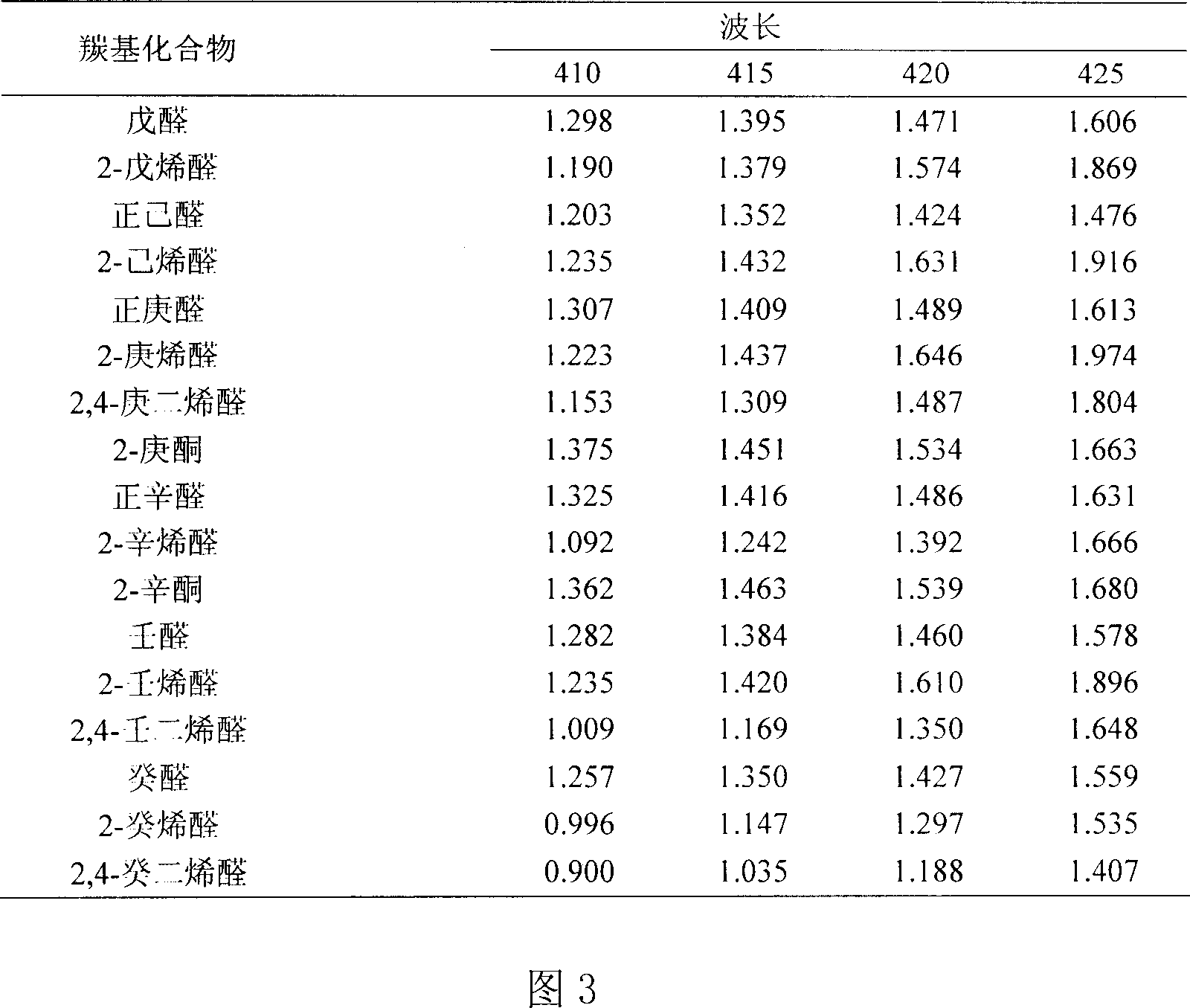
Oxidized and degraded oil and grease contain great amount of harmful carbonyl compound, so that carbonyl compound content in oil and grease becomes one of the important safety indexes. The present invention provides safe, accurate and fast process of measuring carbonyl compound content in oil and grease. The process adopts high safety n-butanol as solvent, and includes the reaction of carbonyl compound in oil and grease and 2, 4-dinitro phenyl hydrazine in acid condition to produce 2, 4- dinitro phenyl hydrazone, the reaction of 2, 4- dinitro phenyl hydrazone in alkaline condition to produce red quinine, centrifugal separation to obtain supernatant, with absorption spectrum in 410-425 nm, and colorimetric quantitative analysis with 2, 4-decadienoic aldehyde as standard matter.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
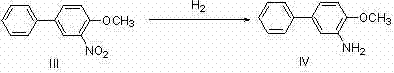
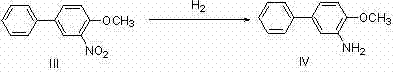
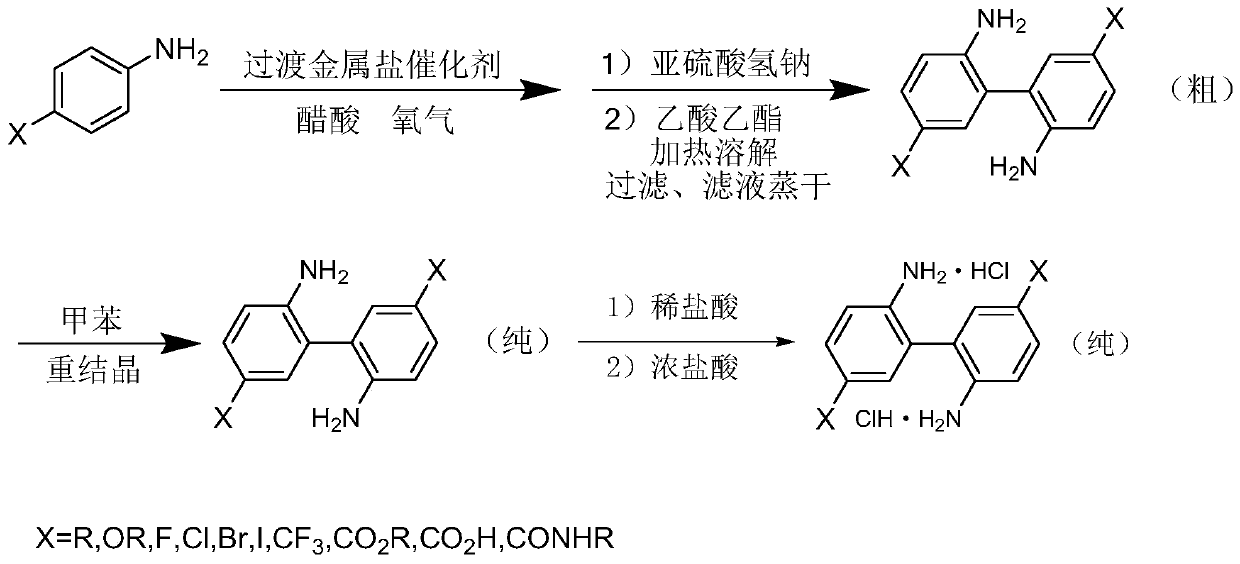
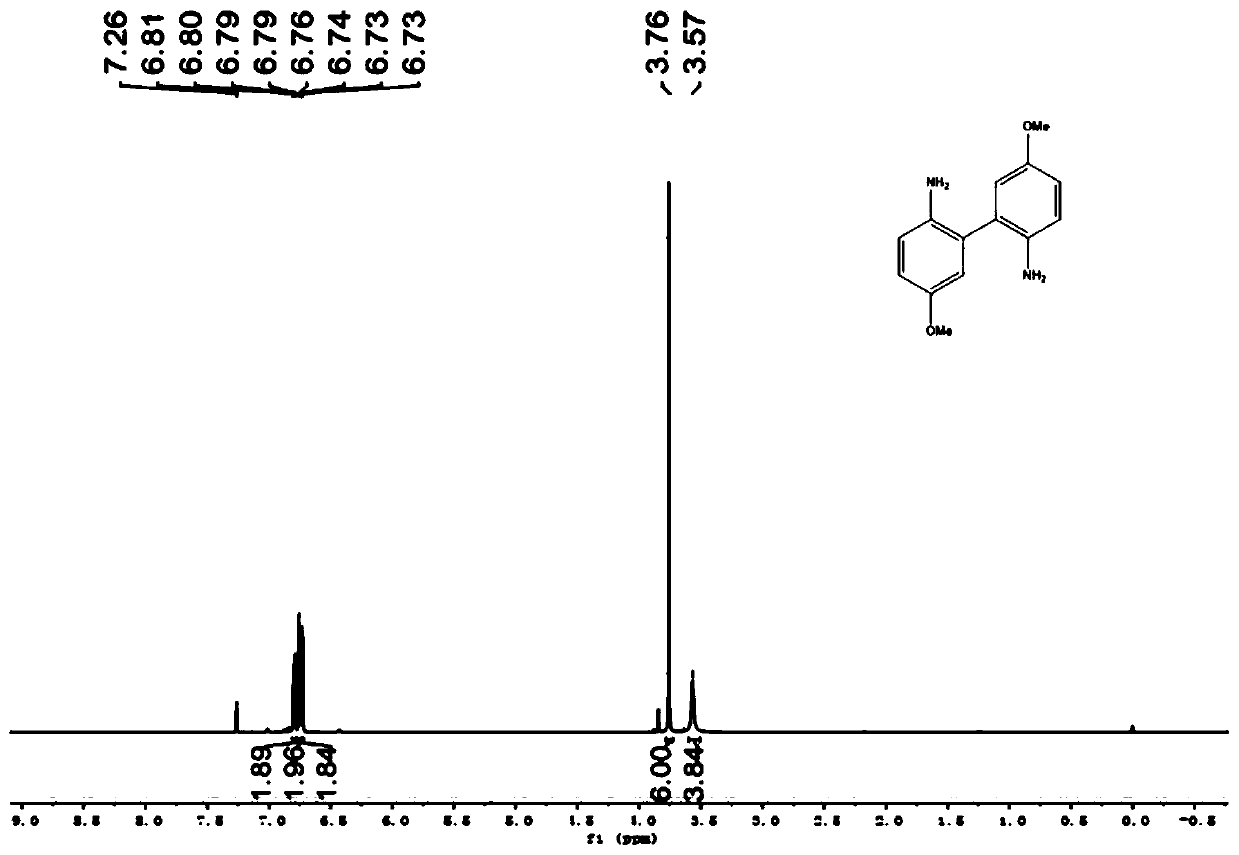
Method for preparing 4,4'-disubstituted-2,2'-diaminodiphenyl and hydrochloride thereof by continuous flow oxidative coupling method
ActiveCN111269129AAccurate temperature controlThe reaction route is reasonableAmino compound purification/separationAmino preparation from aminesRecyclable catalystPtru catalyst
The invention discloses a method for preparing 4,4'-disubstituted-2,2'-diaminodiphenyl and hydrochloride thereof on the basis of 4-substituted nitrobenzene by a reductive coupling method. The method comprises the following steps: in the presence of an organic solvent, a noble metal catalyst and strong alkali, carrying out catalytic hydrogenation by using hydrogen, generating corresponding 1,2-diphenyl hydrazine from the 4-substituted nitrobenzene, then carrying out rearrangement in a hydrochloric acid-ammonium salt mixed solution, and sequentially carrying out pH adjustment, ethyl acetate precipitation, toluene recrystallization, and acidification and acid precipitation treatment to obtain the 4,4'-disubstituted-2,2'-diaminodiphenyl and hydrochloride thereof; the method has the advantagesof cheap and accessible raw materials, simple and convenient operation process, recyclable catalyst, mild reaction conditions and higher yield, and has wide industrial prospects.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
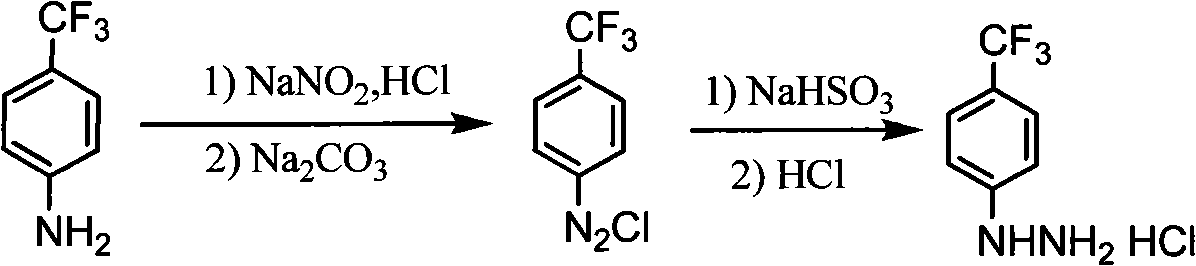
Synthesis method of para-trifluoromethyl phenyl hydrazine hydrochloride
The invention relates to a synthesis method of para-trifluoromethyl phenyl hydrazine hydrochloride, which comprises the following steps: firstly leading para-trifluoromethylaniline and sodium nitrite to carry out diazotization reaction: adding concentrated hydrochloric acid and water into a four-neck flask, and starting stirring; dripping the para-trifluoromethylaniline, generating a larger number of white solids, and controlling the temperature at -5 DEG C-15 DEG C; reducing the temperature to -5 DEG C-5 DEG C, dripping sodium nitrite solution, controlling the temperature at -5 DEG C-15 DEG C, dripping 10-12% of sodium carbonate solution, and adjusting the pH value of diazotization reaction solution to 5-7; further carrying out reduction reaction: adding sodium sulfite solution into the four-neck flask, reducing the temperature to 0-20 DEG C and starting stirring; dripping the diazotization reaction solution into the sodium sulfite solution in batches, keeping the temperature at 0-25 DEG C, and stirring at the room temperature for 1-3h; adding the concentrated hydrochloric acid, and carrying out heating reflux for 1-4h; and reducing the temperature to 0 DEG C-20 DEG C, filtering, drying, and obtaining the para-trifluoromethyl phenyl hydrazine hydrochloride. The yield is greater than 75% and the purity is 97%-99%.
Owner:大连凯飞精细化工有限公司
Metal welding-cutting gas and preparing method thereof
The invention refers to a kind of gas for metal material welding cut and the confecting method. The technology program is: uses liquefied oil gas or liquefied propane as gas and adds in a kind of additives, the composition and the weight proportion of each additive are: cyclohexanone 61-86%, ethanediol methyl ether 13-39%, benzene hydrazine 0-1%. The batch formula of the additive is scientific and reasonable, the additive distributes in the liquid phase or the gas phase evenly, the welding quality reaches or excesses the acetylene gas. The energy consumption of the invention is low, the cost is low, it has no contamination to the environment, the cut surface is flat, the sludge oxide is little, and the slot is narrow.
Owner:杨天作
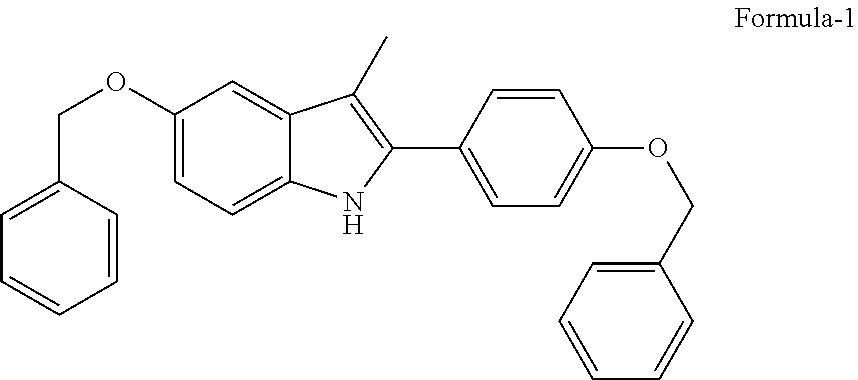
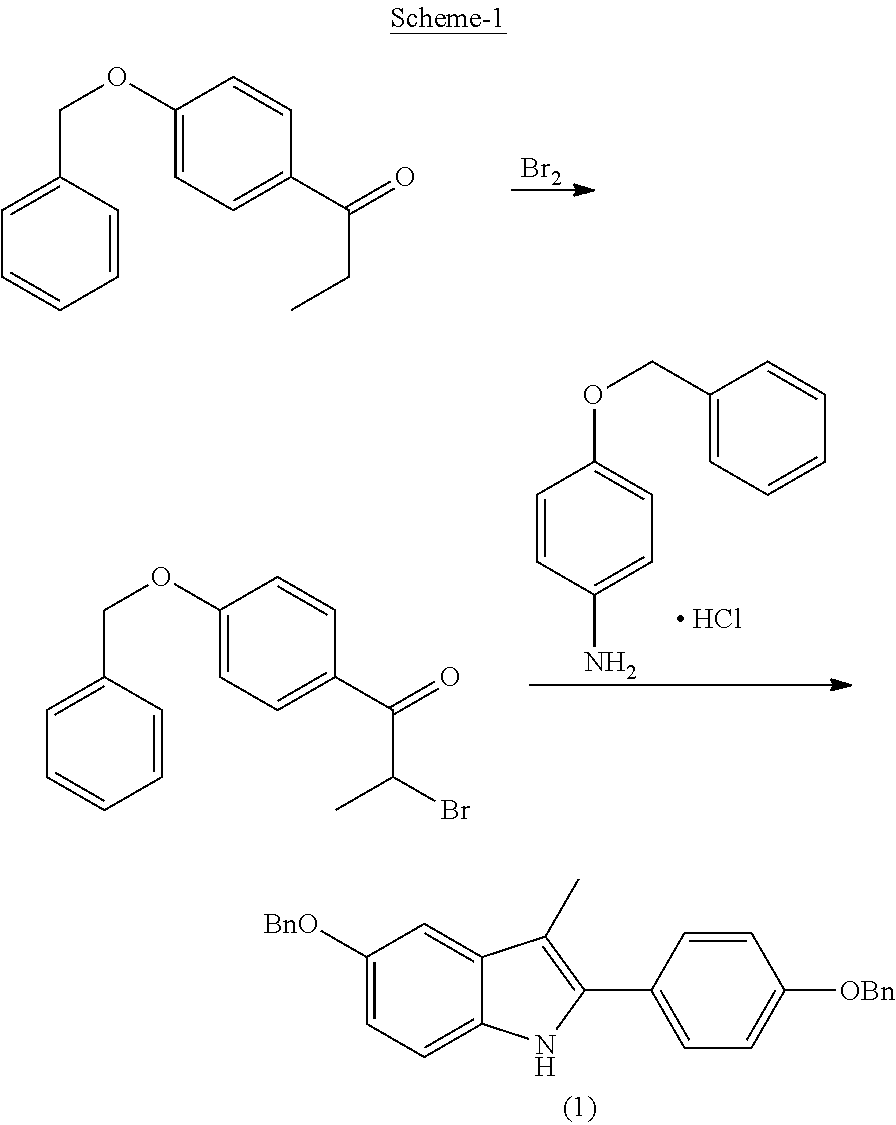
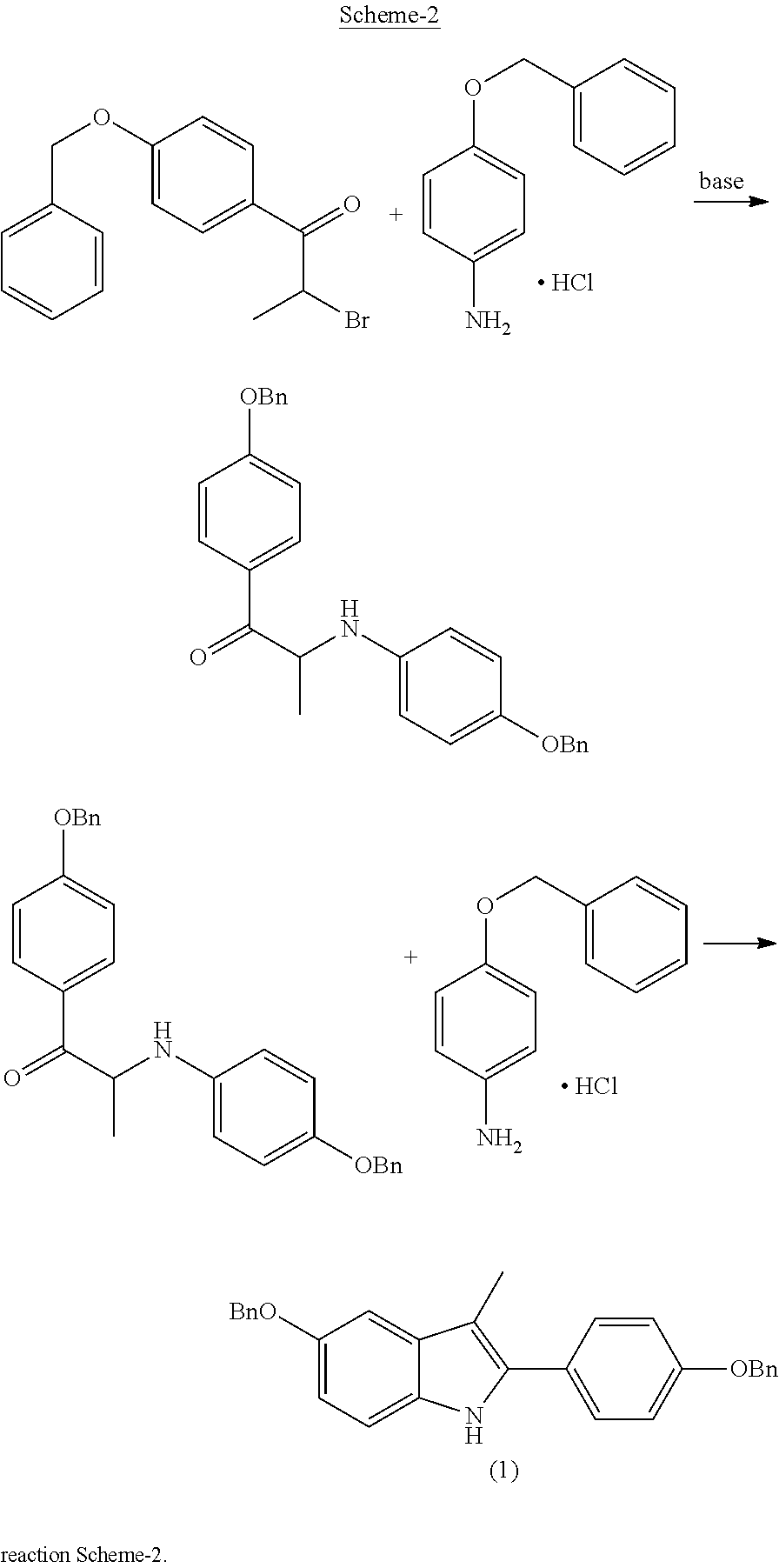
Process for the preparation of 5-benzyloxy-2-(4-benzyloxyphenyl)-3-methyl-1H-indole
The present invention is related to a process for the preparation of 5-benzyloxy-2-(4-benzyloxyphenyl)-3-methyl-1H-Indole (formula-1, a useful intermediate for the synthesis of bazedoxifene) using 4-benzyloxy propiophenone and 4-benzyloxy phenyl hydrazine hydrochloride.
Owner:DIVI S LAB LTD
Bifenazate-containing insecticide composition
Owner:SHAANXI SUNGER ROAD BIO SCI
Manufacturing method for textile
The invention discloses a manufacturing method for a textile. The manufacturing method for the textile comprises the following steps that firstly, desizing and bleaching treatment are carried out on the textile in sequence; secondly, printing and dyeing are carried out on the textile according to designed patterns, and washing and drying are carried out; thirdly, the textile printed with the patterns is evenly coated with phenyl hydrazine sulfonic acid; fourthly, the textile coated with phenyl hydrazine sulfonic acid is subjected to two times of pigment dyeing at 10-20 DEG C; fifthly, the dyed textile is subjected to phenyl hydrazine sulfonic acid removing treatment with hot water at 80-95 DEG C, two times of soaping at 35-45 DEG C and two times of water washing at the room temperature, then drying is carried out, and the textile is obtained. The manufacturing method for the textile has the advantages that the manufacturing method is simple, and the manufactured textile is low in cost, beneficial to market popularization and the like.
Owner:JIANGSU KINGDAY TEXTILE CO LTD
Method for preparing polysubstituted indole from aryl hydrazine and alkyne
The invention discloses a method for preparing polysubstituted indole. The method comprises the following step: carrying out alkyne cyclization reaction based on hydrocarbon chain activation on aryl hydrazine hydrochloride disclosed as Formula II, alkyne disclosed as Formula III, catalyst, alkali and additives to obtain the compound disclosed as Formula I. The accessible raw materials are subjected to on-pot reaction to generate orientating group in situ in the reaction without presetting the orientating group on the substrate. After the cyclization reaction, the orientating group is automatically removed, and the obtained NH indole can be conveniently derived to obtain the N-substituted indole and has favorable compatibility with multiple substituent groups; no additional oxidizer and strong acid or strong alkali are needed in the reaction; the atomic economical efficiency is high; and although the catalyst is not cheap, the addition amount is small.
Owner:TSINGHUA UNIV
Method of using deep eutectic solvent to prepare 2-arylindole compounds
The invention discloses a method of using a deep eutectic solvent to prepare 2-arylindole compounds, and relates to the technical field of organic compound preparation. The deep eutectic solvent is environment-friendly and is taken as the reaction solvent and catalyst. 2-arylindole compounds are prepared through one-step reactions between phenyl hydrazine and substituted acetophenone. The method comprises the following steps: mixing choline chloride (ChCl) and zinc chloride (ZnCl2) according to a mole ratio of 1:4, slowly heating to a temperature of 95 to 100 DEG C, maintaining the temperature, stirring the mixture by magnetic force for 8 to 12 hours to obtain a deep eutectic solvent (ChCl+4ZnCl2); sequentially adding the prepared deep eutectic solvent, phenyl hydrazine, and substituted acetophenone into a reactor according to a mole ratio of 1:1:1, magnetically stirring the mixture for 70 to 90 minutes at a temperature of 120 to 125 DEG C, after reactions, cooling to the room temperature, adding water, stirring, carrying out vacuum suction filtration, washing the filter cake by polyphosphoric acid (10%) until the filter cake becomes neutral to obtain a coarse product, and further recrystallizing the coarse product by ethanol (95%) to obtain a pure crystalline product. The synthesis method is simple, the raw materials are easily available, the cost is low, and the method can be applied to massive production.
Owner:XINZHOU TEACHERS UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com