Method for preparing polysubstituted indole from aryl hydrazine and alkyne
A kind of technology of aryl hydrazine and alkyne, applied in the field of catalytic synthesis of fine chemical products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
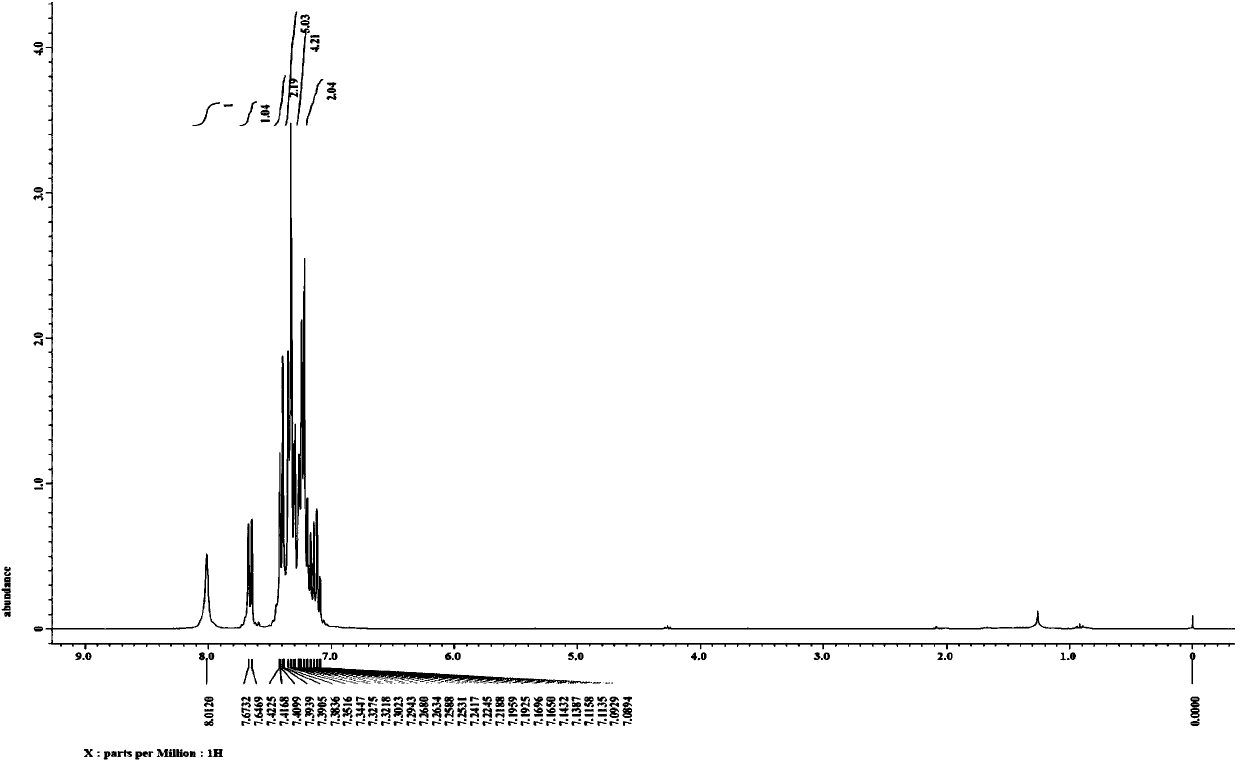
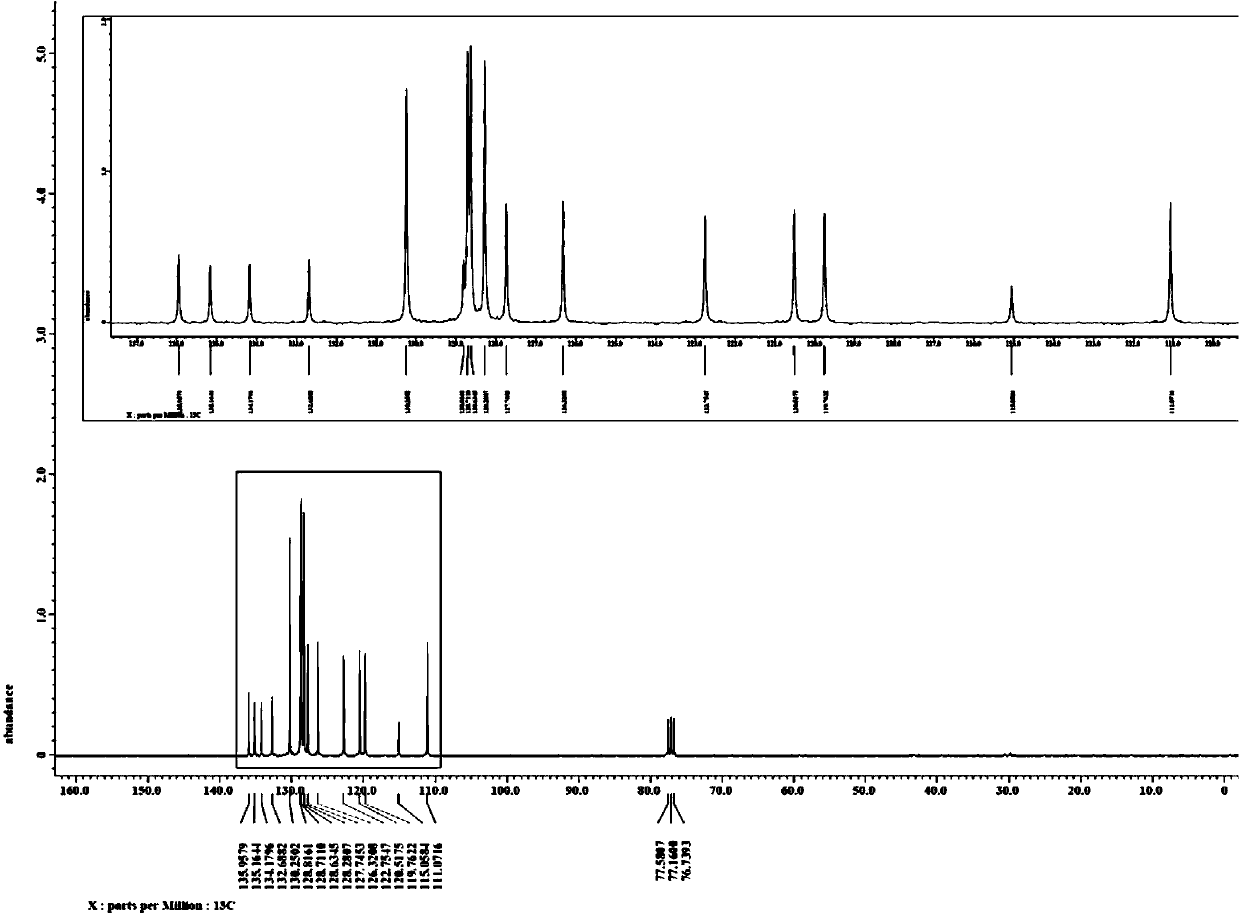
[0049] Weigh successively the phenylhydrazine hydrochloride (R 1 is H) (0.5mmol), 106.9mg belongs to the diphenylacetylene of formula III (R 2 and R 3 Both are phenyl) (0.6mmol), 7.7mg catalyst [Cp*RhCl 2 ] 2 (2.5mol%) and 54.0mg alkali potassium acetate (0.55mmol) in a 25mL sealed tube containing a magnetic stirrer, vacuum-filled with nitrogen for three cycles. Under nitrogen protection, 72.1 mg of additive isobutyraldehyde (1.0 mmol) and 2.5 mL of solvent methanol were added. Seal the sealed tube, put it into an oil bath at 80° C. and stir for 12 hours to carry out the alkyne cyclization reaction based on carbon-hydrogen bond activation. After the reaction was completed, column separation was performed to obtain 111.4 mg of a white solid, and the isolated yield of the target product 2,3-diphenylindole was 83%.
[0050] figure 1 and figure 2 The proton nuclear magnetic resonance spectrum and carbon spectrum of the product prepared in this embodiment are respectively, ...
Embodiment 2
[0052] Weigh successively the p-methylphenylhydrazine hydrochloride (R 1 is methyl) (0.5mmol), 106.9mg belongs to the diphenylacetylene of formula III (R 2 and R 3 Both are phenyl) (0.6mmol), 7.7mg[Cp*RhCl 2 ] 2 (2.5mol%) and 54.0mg potassium acetate (0.55mmol) in a 25mL sealed tube containing a magnetic stirrer, vacuum-filled with nitrogen for three cycles. 72.1 mg of isobutyraldehyde (1.0 mmol) and 2.5 mL of methanol were added under nitrogen protection. Seal the sealed tube, put it into an oil bath at 80°C and stir for 12 hours. After the reaction was completed, column separation was performed to obtain 129.4 mg of a white solid, and the isolated yield of the target product 5-methyl-2,3-diphenylindole was 91%.
Embodiment 3
[0054] Take by weighing 79.3mg m-methylphenylhydrazine hydrochloride (R 1 is methyl) (0.5mmol), 106.9mg belongs to the diphenylacetylene of formula III (R 2 and R 3 Both are phenyl) (0.6mmol), 7.7mg[Cp*RhCl 2 ] 2 (2.5mol%) and 54.0mg potassium acetate (0.55mmol) in a 25mL sealed tube containing a magnetic stirrer, vacuum-filled with nitrogen for three cycles. 72.1 mg of isobutyraldehyde (1.0 mmol) and 2.5 mL of methanol were added under nitrogen protection. Seal the sealed tube, put it into an oil bath at 80°C and stir for 12 hours. After the reaction was completed, column separation was performed to obtain 111.6 mg of a white solid, and the isolated yield of the target product 6-methyl-2,3-diphenylindole was 79%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com