Synthesis method for bifenazate
A technology of bifenazate and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of high cost and difficult industrial production, and achieve the effects of easy post-processing, increased yield, and shortened experimental steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
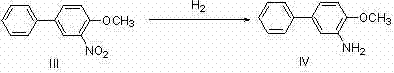
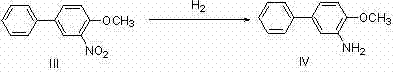
[0031] Example 1: Preparation of Compound Bifenazate (I)
[0032]
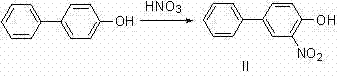
[0033] In a 250ml three-necked flask, 5g (29.4mmol) of p-hydroxybiphenyl was dissolved in 25ml of methyl tert-butyl ether (MTBE), mixed and stirred for 5min, and the temperature was controlled at 30°C. Take 4ml (58.8mmol, 2eq.) of nitric acid mixed with 10ml of MTBE and pour it into the dropping funnel, add dropwise within 1h, and continue to stir for 3h. TLC detects that the reaction ends. The system was poured into 300ml of ice water and stirred for 5 minutes. At this time, there was no dark orange liquid on the surface of the liquid surface, and a large amount of solids were precipitated. They were suction filtered and dried to obtain 5.9 g of yellow solid 3-nitro-4-hydroxybiphenyl (II). The rate is 93%.
[0034]
[0035]
[0036] 5 g (23.2 mmol) of the obtained 3-nitro-4-hydroxybiphenyl solid were mixed with 40 ml of dimethyl carbonate, 8.2 g (1.1 eq.) of tetrabutylammonium bromide (TBAB) and 3.2 ...
Embodiment 2
[0045] Embodiment 2: the preparation of compound bifenazate (I)
[0046]
[0047] In a 250ml three-necked flask, 5g (29.4mmol) of p-hydroxybiphenyl was dissolved in 25ml of methyl tert-butyl ether (MTBE), mixed and stirred for 5min, and the temperature was controlled at 20°C. Take 2ml (29.4mmol, 1eq.) of nitric acid mixed with 10ml of MTBE and pour it into the dropping funnel, add dropwise within 1h, and continue to stir for 3h. TLC detects that the reaction ends. The system was poured into 300ml of ice water and stirred for 5 minutes. At this time, there was no dark orange liquid on the surface of the liquid surface, and a large amount of solids were precipitated. They were suction filtered and dried to obtain 5.9 g of yellow solid 3-nitro-4-hydroxybiphenyl (II). The rate is 80%.
[0048]
[0049]
[0050] 5 g (23.2 mmol) of the obtained 3-nitro-4-hydroxybiphenyl solid were mixed with 30 ml of dimethyl carbonate, 8.2 g (1.1 eq.) of tetrabutylammonium bromide (TBAB) a...
Embodiment 3
[0057] Embodiment 3: the preparation of compound bifenazate (I)
[0058]
[0059] In a 250ml three-necked flask, 5g (29.4mmol) of p-hydroxybiphenyl was dissolved in 25ml of methyl tert-butyl ether (MTBE), mixed and stirred for 5min, and the temperature was controlled at 40°C. Take 2ml (58.8mmol, 2eq.) of nitric acid mixed with 10ml of MTBE and pour it into the dropping funnel. The dropwise addition is completed within 1h, and the stirring is continued for 3h. TLC detects that the reaction ends. The system was poured into 300ml of ice water and stirred for 5 minutes. At this time, there was no dark orange liquid on the surface of the liquid surface, and a large amount of solids were precipitated. They were suction filtered and dried to obtain 5.9 g of yellow solid 3-nitro-4-hydroxybiphenyl (II). The rate is 85%.
[0060]
[0061]
[0062] 5 g (23.2 mmol) of the obtained 3-nitro-4-hydroxybiphenyl solid were mixed with 30 ml of dimethyl carbonate, 8.2 g (1.1 eq.) of tetr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com