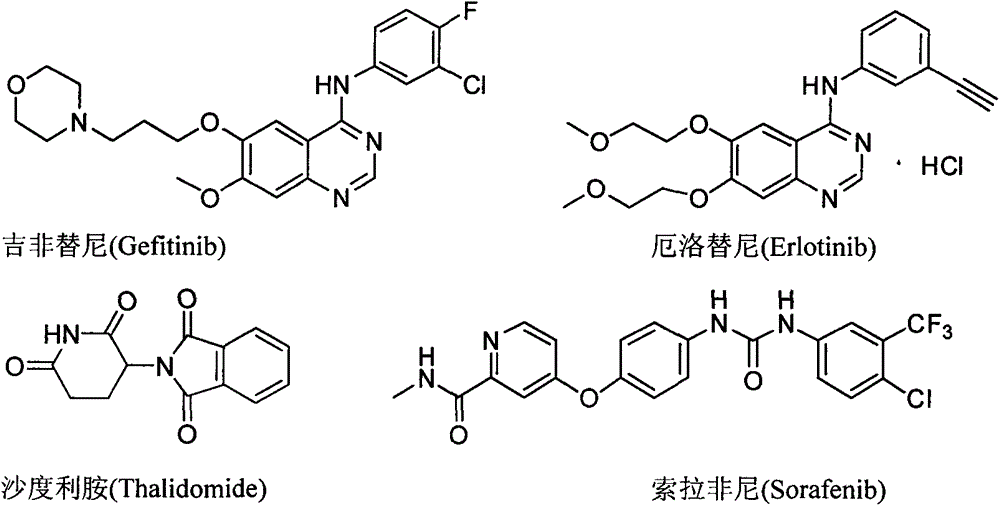
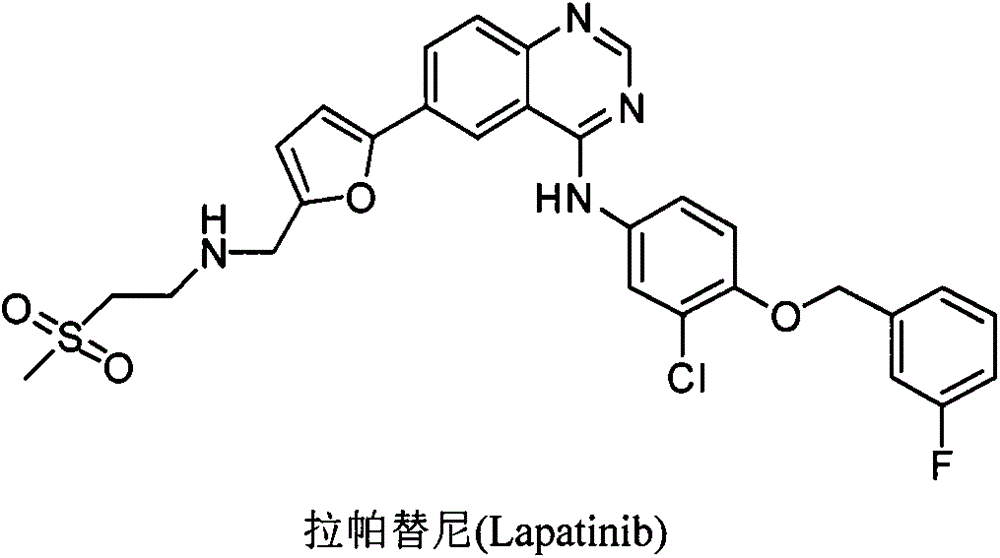
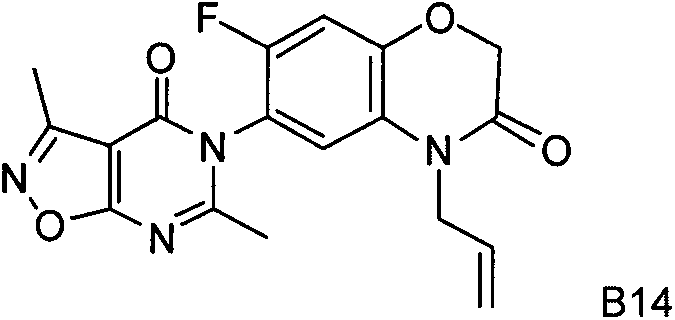
1-substitution phenyl-4-polysubstitution phenyl-5-methylmercapto-1H pyrazole compound with anti-hepatoma activity
A compound, -1H technology, applied in organic chemistry, drug combination, antineoplastic drugs, etc., can solve problems such as high price and drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The synthesis of embodiment 12-chloro-4-fluoro-5-aminophenyl ethyl carbonate 2
[0030] Add 5.26g (20mmol) of ethyl 2-chloro-4-fluoro-5-nitrophenylcarbonate, 10mL of glacial acetic acid and 24mL of water into a 250mL four-neck flask, heat to 40-50°C to dissolve, then add 5.04 g (90mmol) reduced iron powder, stirring vigorously. After the addition, react at 70-80°C for 0.5h, TLC detects that the reaction is complete, stop the reaction, add an appropriate amount of ethyl acetate to the system, keep stirring for 5-10min, filter to remove iron sludge, and wash the filter cake three times with ethyl acetate. Combine the filtrates, wash the filtrate with saturated sodium bicarbonate solution to weak alkalinity, separate with saturated saline, and dry the organic phase with anhydrous sodium sulfate. After filtration and precipitation, the crude product was purified by column chromatography (eluent: petroleum ether / ethyl acetate=20:1 (V:V)) to obtain 3.96 g of an orange-red so...
Embodiment 2
[0033] Synthesis of Example 22-fluoro-4-chloro-5-ethoxyacyloxyphenylfluoroborate diazonium salt 3
[0034] Add 4.66g (20mmol) 2 into a 100mL four-necked flask, slowly add 13.4mL of 18% dilute hydrochloric acid, stir and heat to 40-45°C, after complete dissolution, cool the reaction system to -5°C in an ice-salt bath. Add dropwise a cold solution made of 1.52g (22mmol) sodium nitrite and 2.2mL water, control the temperature below -5°C, after the dropwise addition, continue to stir for 30min, and suction filter under cooling, the filtrate is light yellow. Slowly add 7.6mL of 40% cold fluoboric acid solution dropwise to the filtrate under stirring, and control the temperature below -15°C, a yellow solid is produced. After 15 minutes, the dropwise addition was completed, and the stirring was continued for 45 minutes. Stop stirring, cool at low temperature for 3 hours, filter with suction, wash the filter cake successively with 4 mL of cold dilute fluoroboric acid, 4 mL of 50% eth...
Embodiment 3
[0037] Synthesis of Example 32-chloro-4-fluoro-5-(2-oxopropyl) ethyl phenyl carbonate 4
[0038] In a 250mL two-necked bottle, add 0.27g (1.9mmol) cuprous oxide and 33mL (300mmol) isopropenyl acetate, under the protection of argon, add 5.00g (15mmol) 3 in batches, and control the reaction temperature between 25 and 30°C . After stirring for 0.5 hours, 1.23g (15mmol) of anhydrous sodium acetate was added. After the addition was complete, the temperature was controlled and the reaction was continued for 6h. Suction filtration was performed, and the filter cake was washed with a small amount of dichloromethane. The filtrate was collected and precipitated under reduced pressure, and the crude product was purified by column chromatography (eluent: petroleum ether / ethyl acetate=20:1 (V:V)) to obtain 3.04 g of a yellow solid with a yield of 74.0%. m.p.: 54~56℃.
[0039] 1 H NMR (400MHz, δ: ppm, CDCl 3 ): 7.20 (d, 3 J H-F =8.8Hz, 1H, Ar-H), 7.09(d, 4 J H-F =6.7Hz, 1H, Ar-H), 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com