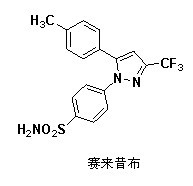
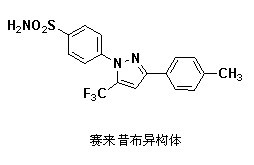
Preparation method for celecoxib isomer
A technology of celecoxib and isomers, which is applied in the field of preparation of celecoxib isomers, can solve the problem of low content and achieve the effects of high yield, high selectivity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
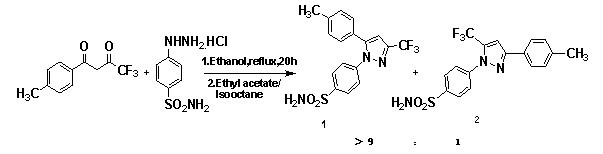
[0022] Participate in Figure 4 , the preparation method of celecoxib isomer of the present invention comprises the following steps:
[0023] Step (1): In a round bottom flask, dissolve 46.0 g of 4,4,4-trifluoro-1-(4-methylphenyl)-1,3-butanedione in 300 mL of ethanol, and add 18.4 g of methoxylamine hydrochloride, and then heated to reflux for 8-10 h under stirring, followed by TLC spot plate. After the reaction was completed, the solvent was removed in vacuo, and 46.6 g of crude product 4,4,4-trifluoro-3-methoxyimino-1-(4-methylphenyl)-1-butanone was obtained by eluting with petroleum ether as a solvent. The yield is 90%.
[0024] Step (2): In a round bottom flask, add 46.6 g of crude product 4,4,4-trifluoro-3-methoxyimino-1-(4-methylphenyl)-1-butanone obtained in step (1) and 48.4 g of p-sulfamoylphenylhydrazine hydrochloride were dissolved in 300 mL of ethyl acetate (or petroleum ether) and 15 mL of acetic acid (also hydrochloric acid or sulfuric acid), then heated to re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com