Preparation method of 5-amino-3-cyano-1-(2, 6-dichloro-4-trifluoromethylphenyl)pyrazole
A technology of trifluoromethylphenyl and trifluoromethylaniline, which is applied in the field of preparation of 5-amino-3-cyano-1-pyrazole, can solve the problem that the product content can only reach 98%, and the production risk coefficient can be quantified. Large, poor chlorination reaction selectivity, etc., to achieve the effect of high product yield, low equipment requirements, and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of intermediate ring compounds
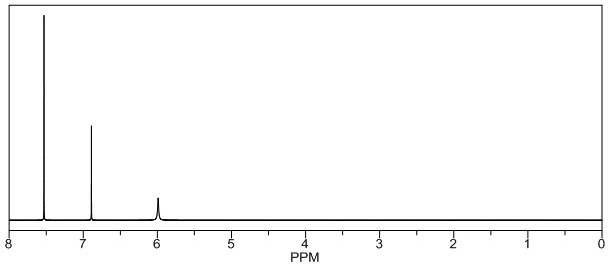
[0040] Add 167.2g (1.1mol) of ethyl 2,3-dicyanopropionate, 500g ethanol, and 200g 30% hydrochloric acid into a 2000mL four-necked reaction flask, start stirring, cool down to 0°C, and start to drop trifluoro Mixed solution of 161g (1.0mol) of methylaniline and 160g of ethanol and 195g of sodium nitrite solution (sodium nitrite: 79g, water: 116g), control the rate of addition, and finish dropping at the same time for about 6 hours. For 4 hours, the sample was controlled in the middle, and the purity of trifluoromethylaniline was 0.3% by HPLC. Add 50g of sodium bisulfite solution (sodium bisulfite: 20g, water: 30g) to quench the reaction. After the reaction, dilute with 15% 120g of ammonia water was used to adjust the pH=5, the dropping time was 4h, the temperature was 12°C, and then the temperature was kept at 15°C for 2h. The central control, the HPLC detection purity of the ring compound was 99.2%, and the solvent ethanol was ...
Embodiment 2
[0042] Synthesis of intermediate ring compounds
[0043] Add 197.6g (1.3mol) of ethyl 2,3-dicyanopropionate, 500g of ethanol, and 250g of 30% hydrochloric acid into a 2000mL four-necked reaction flask, start stirring, cool down to -5°C, and start to dropwise add three Mixed solution of 161g (1.0mol) of fluoromethylaniline and 160g of ethanol and 250g of sodium nitrite solution (sodium nitrite: 100g, water: 150g), control the rate of addition, and drop it at the same time for about 8 hours, and drop it at 5°C Insulated reaction for 4 hours, sampling control, the purity of trifluoromethylaniline HPLC detection is 0.2%, adding 50g of sodium bisulfite solution (sodium bisulfite: 20g, water: 30g) to quench the reaction, after the reaction is completed, use 15 % diluted ammonia water 110g to adjust the pH=4, the dropping time is 3h, the temperature is 18°C, and then continue to keep warm at 15°C for 2h, sampling and testing, the purity of the ring compound is 99.46% by HPLC, the sol...
Embodiment 3
[0046] Synthesis of 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)pyrazole
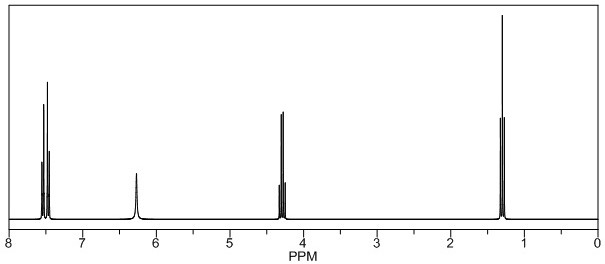
[0047] Add 330g (content: 96.95%) of the intermediate cyclic compound of Example 1 into a 2000mL four-necked reaction flask, 1350g dichloroethane, 8g (0.04878mol) of azobisisobutyronitrile, stir, and start to pass through at 15°C. Chlorine gas 281g (3.96mol), after about 4 hours, the temperature did not exceed 45°C during the chlorine pass, then began to heat up to 80°C and continued to keep warm for 4 hours, sampling and testing, the purity of the intermediate cyclic compound was 0.2% by HPLC, stop the reaction , add 200g of water to stir, let stand to separate and separate, adjust the pH=10 with 150g of 15% dilute ammonia water in the oil phase, add the time for 2h, the temperature is 25°C, continue the reaction at 25°C for 4h, take a sample for detection, and the purity of the product by HPLC is 98.49 %, start to recover dichloroethane under reduced pressure; after the recovery, add 650g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com