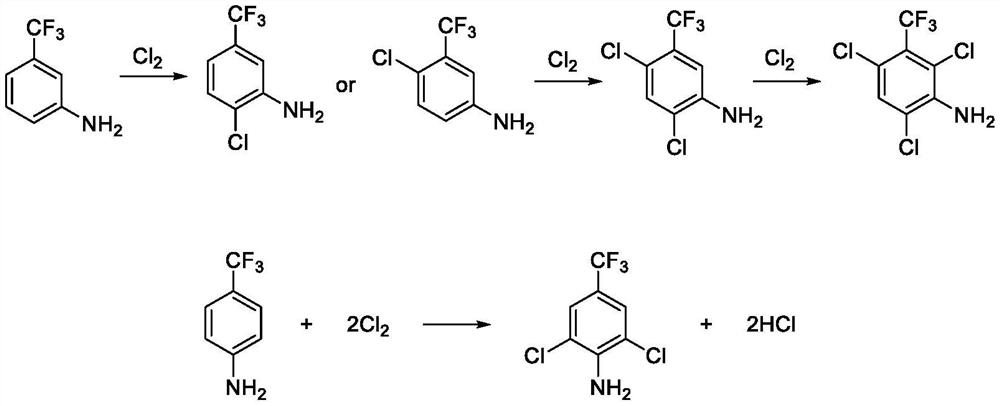
Method for preparing 2, 6-dichloro-4-trifluoromethylaniline
A technology of trifluoromethylaniline and dichloride, which is applied in chemical instruments and methods, preparation of amino compounds, preparation of organic compounds, etc., can solve the problems such as difficulty in handling the rectifying kettle, and achieves less generation of three wastes, simple process, The effect of short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 1000g hydrochloric acid (8%), 80g m-trifluoromethylaniline rectification raffinate (p-trifluoromethylaniline 72.5%, m-trifluoromethylaniline 25.5%) in the reaction bottle, stir, and the raffinate is all dissolved . After the temperature was raised to 40°C, chlorine gas was introduced, and the reaction temperature was maintained at 50°C to 60°C. The reaction was monitored by GC, and the reaction was stopped when the content of 2,4-dichloro-5-trifluoromethylaniline was equal to 0.18%. Neither methylaniline nor 2-chloro-4-trifluoromethylaniline was detected, and the reaction time was 45 minutes. The reaction solution was statically separated, and the lower organic phase was washed with 100mL lye (2%), then evaporated under reduced pressure to remove water, and then rectified under reduced pressure to obtain 2,6-dichloro-4-trifluoromethylaniline 73.2 g, the GC purity is 99.74%, and the yield is 88.4% based on p-trifluoromethylaniline. The above percentages are all per...
Embodiment 2
[0036] Add 726g hydrochloric acid (12%), 67g m-trifluoromethylaniline rectification raffinate (p-trifluoromethylaniline 69.6%, m-trifluoromethylaniline 28.4%) in the reaction bottle, stir and raffinate is all dissolved, After the temperature was raised to 40°C, chlorine gas was introduced, and the reaction temperature was maintained at 50°C-60°C. The reaction was detected by GC, and the reaction was stopped when the content of 2,4-dichloro-5-trifluoromethylaniline was equal to 0.23%. Neither methylaniline nor 2-chloro-4-trifluoromethylaniline was detected, and the reaction time was 38 minutes. The reaction solution was statically separated, and the lower organic phase was washed with 100mL lye (2%), then evaporated under reduced pressure to remove water, and then rectified under reduced pressure to obtain 2,6-dichloro-4-trifluoromethylaniline 58.4 g, the GC purity is 99.63%, and the yield is 87.6% based on p-trifluoromethylaniline. The above percentages are all percentages by...
Embodiment 3
[0038] Add 1600g hydrochloric acid (20%), 160g m-trifluoromethylaniline rectification raffinate (p-trifluoromethylaniline 71.2%, m-trifluoromethylaniline 26.9%) in the reaction bottle, stir and raffinate is all dissolved, After the temperature was raised to 40°C, chlorine gas was introduced, and the reaction temperature was maintained at 50°C-60°C. The reaction was detected by GC, and the reaction was stopped when the content of 2,4-dichloro-5-trifluoromethylaniline was equal to 0.11%. Neither methylaniline nor 2-chloro-4-trifluoromethylaniline was detected, and the reaction time was 55 minutes. The reaction solution was statically separated, and the lower organic phase was washed with 100mL lye (2%), then evaporated under reduced pressure to remove water, and then rectified under reduced pressure to obtain 2,6-dichloro-4-trifluoromethylaniline 141.8 g, the GC purity is 99.77%, and the yield is 86.9% based on p-trifluoromethylaniline. The above percentages are all percentages...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com