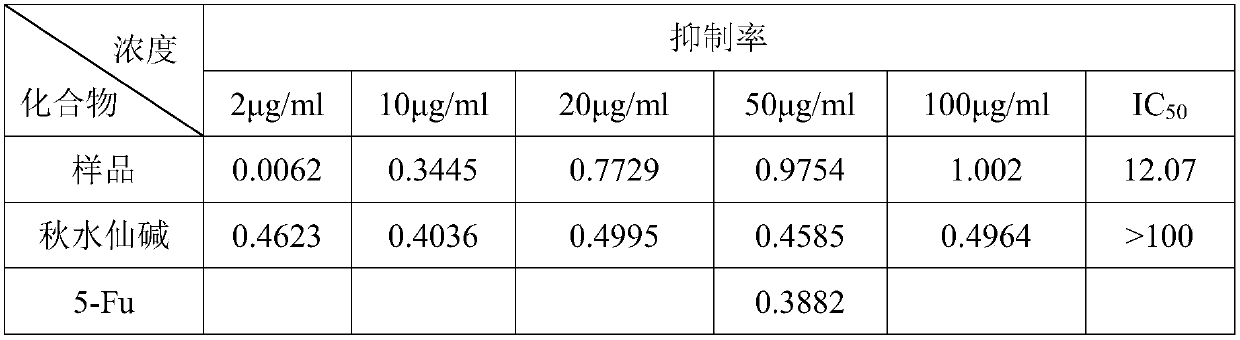
Synthesis method of triphenyl-4-(trifluoromethylbenzamide)butylphosphonium chloride and application of triphenyl-4-(trifluoromethylbenzamide)butylphosphonium chloride in antitumor drugs
A technology of trifluoromethylbenzamide group and trifluoromethylaniline, which is applied in the synthesis field of triphenyl-4-butylphosphonium chloride, can solve the problems of difficult control, low product purity of quaternary phosphonium salt, and poor product purity. It can reduce the excessive proportion of side reactions, overcome the harsh synthesis conditions, and reduce the reaction temperature.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
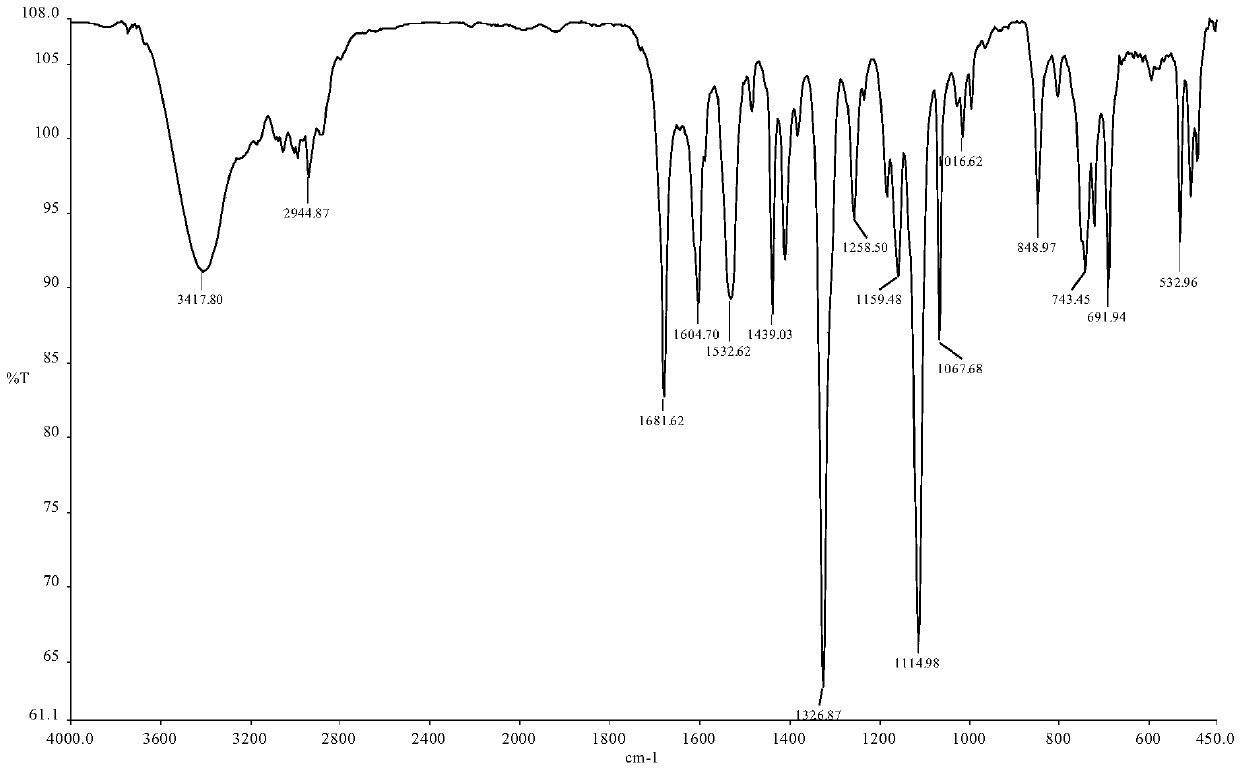
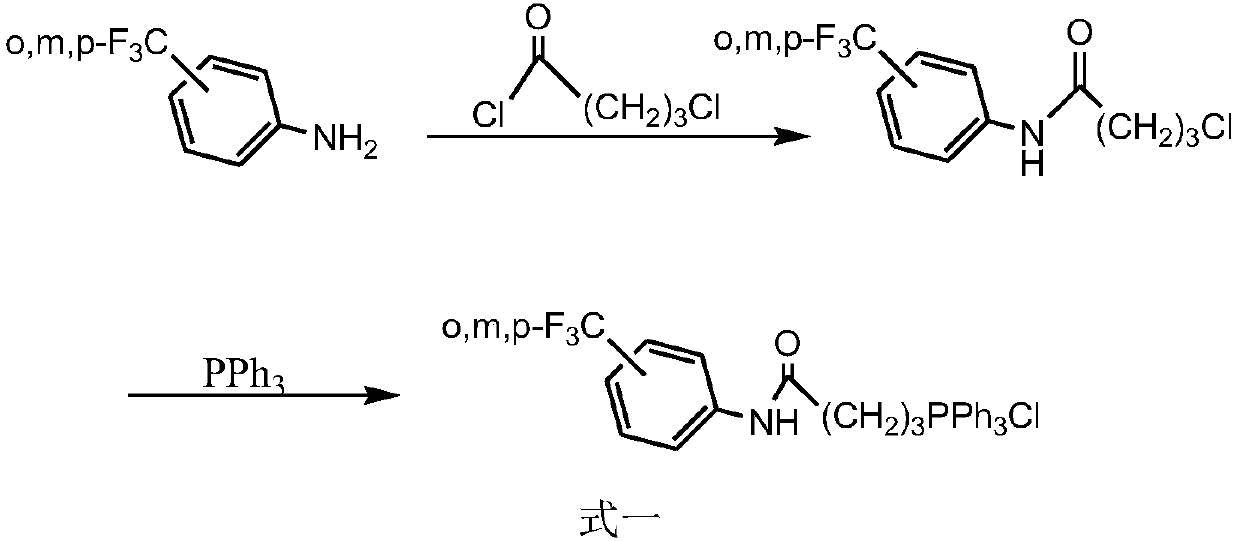
[0036] A kind of synthetic method of triphenyl-4-(m-trifluoromethylbenzamido) butylphosphonium chloride of the present embodiment comprises the following steps:
[0037] (1) Synthesis of N-trifluoromethylphenyl-4-chlorobutanamide
[0038] Dissolve 0.82g (5mmol) m-trifluoromethylaniline in 15ml chloroform in the reaction flask, then add 0.50g (5mmol) triethylamine, control the temperature in an ice-water bath, stir, then slowly add 4 -Chlorobutyryl chloride 0.84g (6mmol), after reacting for 20min, remove the ice bath, continue to heat and reflux, react for 2.5h, TLC monitors the reaction process, until the reaction is complete, use saturated Na 2 CO 3 After the solution was washed, it was washed with saturated brine, and then extracted twice with 15ml chloroform, the organic phases were combined and then washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure, separated by column chromatography, and separated wit...
Embodiment 2
[0042] A kind of synthetic method of triphenyl-4-(m-trifluoromethylbenzamido) butylphosphonium chloride of the present embodiment comprises the following steps:
[0043] (1) Synthesis of N-trifluoromethylphenyl-4-chlorobutanamide
[0044] Dissolve 0.82g (5mmol) m-trifluoromethylaniline in 15ml chloroform in the reaction flask, then add 0.53g (5mmol) sodium carbonate solid, control the temperature in an ice-water bath, stir, then slowly add 4 -Chlorobutyryl chloride 0.84g (6mmol), after reacting for 30min, remove the ice bath, continue to heat and reflux, react for 5h, TLC monitors the reaction progress, until the reaction is complete, use saturated Na 2 CO 3 solution, then washed with saturated brine, extracted twice with 15ml chloroform respectively, the organic phases were combined and then washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure, separated by column chromatography, and separated with solvent...
Embodiment 3
[0048] A kind of synthetic method of triphenyl-4-(o-trifluoromethylbenzamido) butylphosphonium chloride of the present embodiment comprises the following steps:
[0049] (1) Synthesis of N-o-trifluoromethylphenyl-4-chlorobutanamide
[0050] Dissolve 0.33 g (2 mmol) of o-trifluoromethylaniline in 10 ml of chloroform in a reaction flask, add 0.20 g (2 mmol) of triethylamine, control the temperature in an ice-water bath, stir, and slowly add 4- Chlorobutyryl chloride 0.36 g (2.6 mmol). After reacting for 20min, remove the ice bath, continue to heat and reflux, react for 2.5h, TLC monitors the reaction process, until the reaction is complete, use saturated Na 2 CO 3 solution, then washed with saturated brine, extracted twice with 15ml chloroform respectively, the organic phases were combined and then washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure, separated by column chromatography, and diethyl ether and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com