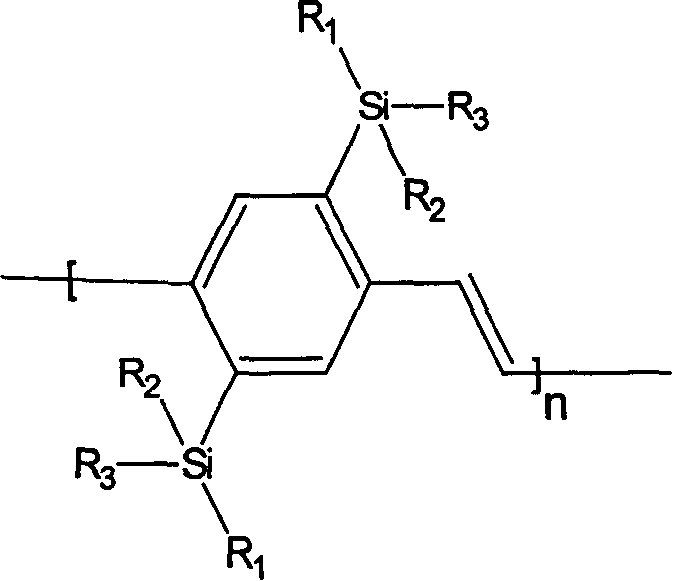
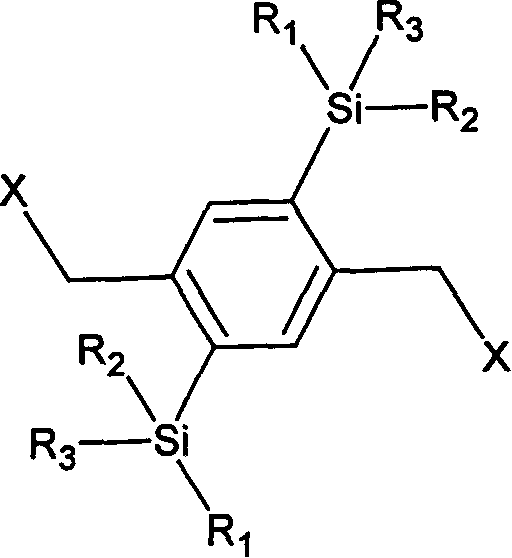
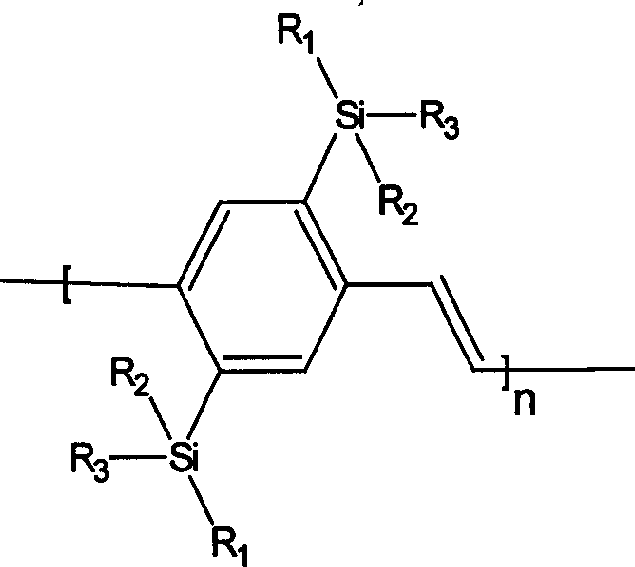
Silicon group containing 1, 4-phenylene vinylidene and its preparation method and use
A technology of phenylene vinylene and disilylbenzene, which is applied in 1 field, can solve the problems of increasing the complexity of device manufacturing and affecting device performance, and achieve good carrier fluid injection and transmission capabilities, high luminous efficiency, and good film formation performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Synthesis of 2,5-bis(bismethoxyoctylsilyl)-PPV: the structural formula is as follows:
[0037]
[0038] (1) Synthesis of 1,4-bis(bismethoxyoctylsilyl)benzene: 10.0 grams (42mmol) of p-dibromobenzene was dissolved in 100ml of anhydrous tetrahydrofuran, and N 2 For protection, 50ml (100mmol) of 2M freshly prepared n-butyllithium tetrahydrofuran solution was slowly added dropwise at -78°C, and after maintaining the temperature for 3 hours, 20g (84mmol) dimethoxyoctane was slowly added dropwise at -78°C Chlorosilane, after 2 hours of reaction, the temperature was slowly raised to room temperature within 3 hours, and the reaction was completed at room temperature for 6 hours. The reactant was washed with water, dried and distilled under reduced pressure to obtain the product. Boiling point: 173°C / 0.2mmHg.
[0039] The analytical results of the product are as follows:
[0040] 1 H NMR (R3DR3l3 ): δ (ppm) 7.08 (4H, singlet, R1r-H), 1.45-1.15 (24H, multiplet), ...
Embodiment 2
[0049] Example 2 Synthesis of 2,5-bis(methoxybutoxyoctylsilyl)-PPV: the structural formula is as follows:
[0050]
[0051] (1) Synthesis of 1,4-bis(methoxybutoxyoctylsilyl)benzene: 10.0 grams of p-dibromobenzene (42mmol) were dissolved in 500ml of anhydrous tetrahydrofuran, passed into N 2 For protection, 100ml (200mmol) of 2M freshly prepared n-propyllithium tetrahydrofuran solution was slowly added dropwise at -70°C, and the reaction was maintained at this temperature for 3 hours. 40 g (142 mmol) of methoxybutoxyoctylchlorosilane was slowly added dropwise at -70°C, and after 2 hours of reaction, the temperature was slowly raised to room temperature within 3 hours, and the reaction was completed at room temperature for 12 hours. The reactant was washed with water, dried and distilled under reduced pressure to obtain the product. Boiling point: 181°C / 0.2mmHg.
[0052] The analytical results of the product are as follows:
[0053] 1 H NMR (R3DR3l 3 ): δ (ppm) 7.06 (4H,...
Embodiment 3
[0062] Example 3 Synthesis of 2,5-bis(methyloctyloctadecylsilyl)-PPV: the structural formula is as follows:
[0063]
[0064] (1) Synthesis of 1,4-bis(methyloctyloctadecylsilyl)benzene: 10.0 grams (42mmol) of p-dibromobenzene are dissolved in 200ml of anhydrous tetrahydrofuran, and N 2 For protection, 120ml (240mmol) of fresh 2M isooctyllithium tetrahydrofuran solution was slowly added dropwise at -80°C, and the reaction was maintained at this temperature for 8 hours. Slowly add 130 g (292 mmol) of methyloctyloctadecylchlorosilane dropwise at -80°C. After 5 hours of reaction, the temperature is slowly raised to room temperature within 3 hours, and the reaction is completed at room temperature for 12 hours. The reactant was washed with water, dried and distilled under reduced pressure to obtain the product. Boiling point: 168°C / 0.1mmHg.
[0065] The analytical results of the product are as follows:
[0066] 1 H NMR (R3DR3l 3 ): δ (ppm) 7.23 (4H, singlet, R1r-H), 1.50-1....
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com