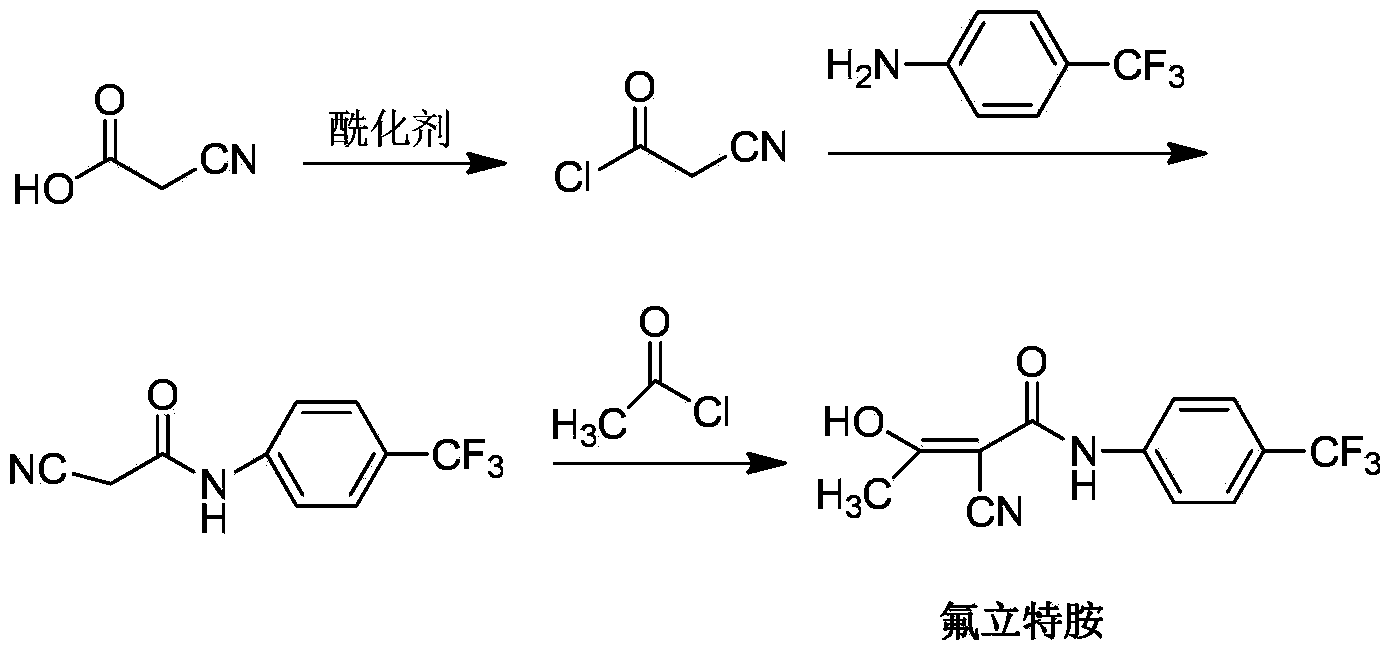
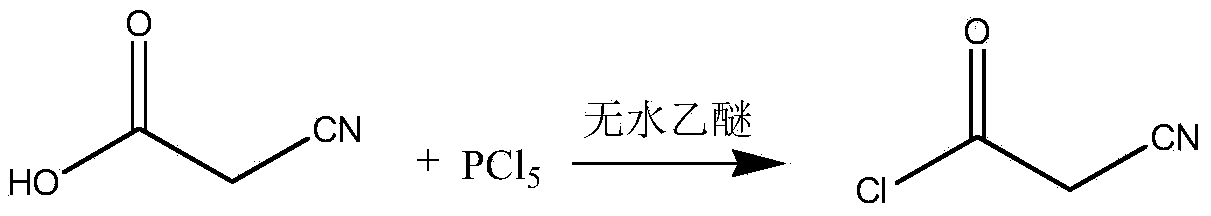Preparation method of teriflunomide
A technology of fluritamide and acetamide, applied in the field of preparation of fluritamide (Teriflunomide), can solve the problems of low yield, long route, unfavorable for actual production and the like, and achieves high yield, easy-to-obtain raw materials, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Preparation of intermediate cyanoacetyl chloride: In a three-necked flask, add 1.7g cyanoacetic acid (0.02mol), anhydrous ether 20mL, stir and dissolve, then add 8.3g phosphorus pentachloride (0.04 mol), at the beginning of addition, the reaction temperature rose rapidly, and then gradually stabilized at 10°C. After the addition was complete, stir for 1 hour. The reaction equation is as follows:
[0015]
[0016] Then the solvent was evaporated under reduced pressure, and the solution turned yellow. 20 mL of toluene was added, and the solvent was removed under reduced pressure to obtain a brown liquid. The intermediate was not purified and was used for future use.
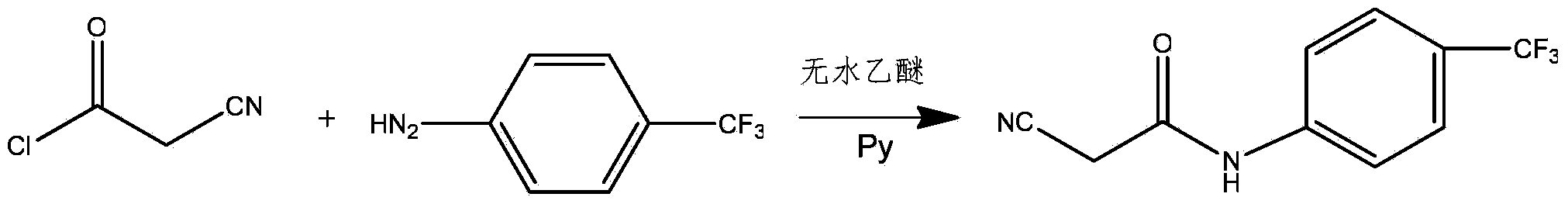
[0017] Preparation of 2-cyano-N-(4-trifluoromethyl-phenyl)-acetamide: Add 2.254g p-trifluoromethylaniline (0.014mol) and 10ml (0.126mol) of pyridine to the reaction flask, and anhydrous Take 20ml of diethyl ether, cool in an ice bath, add 1.22g (0.012mol) of cyanoacetyl chloride obtained in the previous...
Embodiment 2
[0025] The preparation of the cyanoacetyl chloride of embodiment 2 and the preparation of 2-cyano group-N-(4-trifluoromethyl-phenyl)-acetamide are the same as embodiment 1, difference is: the intermediate prepared in embodiment 1 Add 0.8g (0.0035mol) of 2-cyano-N-(4-trifluoromethyl-phenyl)-acetamide to 10mL of acetonitrile, and add 1.42g (0.0141mol) of triethylamine under ice-bath conditions to complete the addition After cooling to -15°C, 0.444 g of acetyl chloride (0.0056 mol) was added dropwise. The reaction was violently exothermic, and the solution gradually turned reddish brown. After the addition of acetyl chloride, the ice bath was removed and the temperature was slowly raised to room temperature.
[0026] After reacting for 5 hours, the solution gradually turned light yellow, and was subjected to suction filtration, and the filter cake of the suction filtration was detected by TLC to have no product. The filtrate was adjusted to pH = 2 with 10% hydrochloric acid. Du...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com