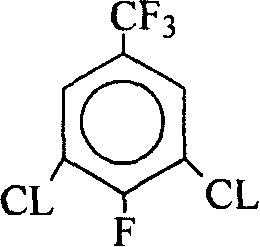
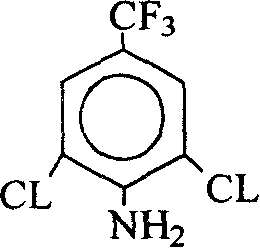
Method for producing 2,6-dichloro-4-trifluoromethylaniline
A technology of trifluoromethylaniline and trichlorobenzotrifluoride, which is applied to the preparation of amino compounds, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of difficult purification of products, corrosion of equipment, expensive raw materials, etc., and achieve reduction Low production cost, low equipment corrosion, and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 200 grams of 3,4,5-trichlorobenzotrifluoride, 200 grams of potassium fluoride and 500 milliliters of sulfolane into a 1L three-neck reaction flask equipped with a stirrer, reflux condenser and temperature, then heat to 190°C and keep warm After stirring for 20 hours, it was cooled to 100°C.
[0021] 200 g of crude product (containing about 10% sulfolane) were distilled out under a pressure of 0.01 MPa. Then the temperature was raised to 150° C. to recover sulfolane under reduced pressure (it can be directly used in the next batch of reactions).
[0022] 200 g of crude product 3,5-dichloro-4-fluorobenzotrifluoride was directly used in the next step of ammoniation reaction.
Embodiment 2
[0024] Add 200 grams of crude product 3,5-dichloro-4-fluorobenzotrifluoride and 300 milliliters (0.892 g / ml) of 30% ammonia water in Example 1 into a 1L autoclave, heat up to 150° C., and then keep stirring for 24 hours. Cooling, discharging, layering, separate the lower oil layer (the water layer can be used for the next batch of reactions); the oil layer is subjected to vacuum distillation to obtain 153 grams (97%) of 2,6-dichloro-p-trifluoromethyl Aniline (melting point: 32-34 degrees).
Embodiment 3
[0026] Add 100 grams of 3,5-dichloro-4-fluorobenzotrifluorotoluene crude product and 30 grams of liquid ammonia prepared by the method in Example 1 into a 300-ml autoclave, then heat up to 140 ° C, then keep stirring for 6 hours, and then remove the residual ammonia Discharge (water absorption), cool the discharge, and rectify under reduced pressure to obtain 78 grams (97.4%) of 2,6-dichloro-p-trifluoromethylaniline (melting point: 32-34 degrees).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com