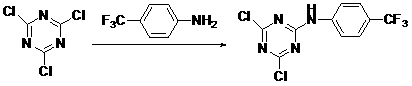
2,4-dichloro-6-(4-aminobenzotrifluoride)-1,3,5-triazine and preparation method and application thereof
A technology of trifluoromethylanilino and trifluoromethylaniline, applied to 2,4-dichloro-6-(4-trifluoromethylanilino)-1,3,5-triazine and its preparation and application fields to achieve the effect of great development potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Synthesis of 2,4-dichloro-6-(4-trifluoromethylanilino)-1,3,5-triazine
[0018] Add 1.9 g (10 mmol) of cyanuric chloride to a 150 mL three-neck flask, add 30 mL of acetone, stir to dissolve, cool to 0 °C in an ice bath, slowly add 1.6 g (10 mmol) of p-trifluoromethylaniline dropwise 20 ml of acetone solution was added after 10 min, and the reaction was stirred at 0°C for 3 hours. After the completion of the reaction, the pH value of the reaction system was adjusted to 6 with 10% aqueous sodium carbonate solution, and a white solid was left to separate out. After suction filtration, the filter cake was recrystallized with ethanol and water, and after drying, 2.47 g of white solid was obtained. Yield: 80%, mp: 155~157℃, 1 H NMR (DMSO, 400 MHz) δ:7.73~7.75 (d, J=8.56 MHz, 2H, Ar H ), δ: 7.84~7.86 (d, J=8.68 MHz, 2H, Ar H ), δ: 11.47 (s, 1H, N H ); MS(ESI):309(M + ).
Embodiment 2
[0020] The bactericidal activity of the solvent was determined by the colony diameter in the growth rate method and the toxic medium culture method. On the aseptic operating table, first prepare a certain concentration of the solvent solution to be tested, then use a pipette gun to draw 1mL of the prepared solvent solution into a 7.5cm petri dish, use sterilized distilled water as a blank control, and then use sterilized Inject 9mL of melted PDA medium into a 10mL glass syringe, mix the liquid medicine and the medium evenly in the petri dish, and after cooling to room temperature, inoculate brown spot bacteria with a diameter of 5mm in the middle of the petri dish. Each treatment Do 3 repetitions, and finally put the inoculated petri dish into a constant temperature incubator at 28°C for about 30-40 hours. When the blank control is nearly full, use a ruler to measure the diameter of the colony. Measure 2 times, take the average value, and finally calculate the bacteriostatic r...
Embodiment 3
[0026] The bactericidal activity of the solvent was determined by the colony diameter in the growth rate method and the toxic medium culture method. On the aseptic operating table, first prepare a certain concentration of the solvent solution to be tested, then use a pipette gun to draw 1mL of the prepared solvent solution into a 7.5cm petri dish, use sterilized distilled water as a blank control, and then use sterilized Inject 9mL of melted PDA medium into a 10mL glass syringe, mix the liquid medicine and medium evenly in the petri dish, and after cooling to room temperature, inoculate cotton wilt bacteria with a diameter of 5mm in the middle of the petri dish. Repeat 3 times, and finally put the inoculated petri dish into a constant temperature incubator at 28°C and incubate for 40-60 hours. When the blank control is nearly full, measure the diameter of the colony with a ruler, and measure each petri dish with the cross method 2 times, take the average value, and finally cal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com