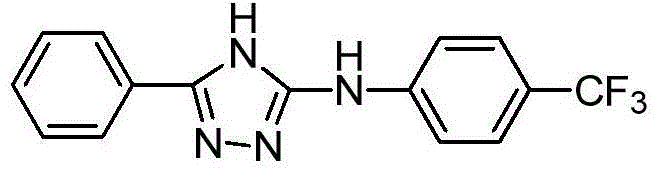
3-phenyl-5-(4-trifluoromethyl phenylamino)-4H-1,2,4-triazole as well as synthetic process and application thereof
A trifluoromethylaniline-based, synthetic process technology, applied in the fields of application, botanical equipment and methods, insecticides, etc., can solve the problem that the skeleton structure is not a triazole ring, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016]
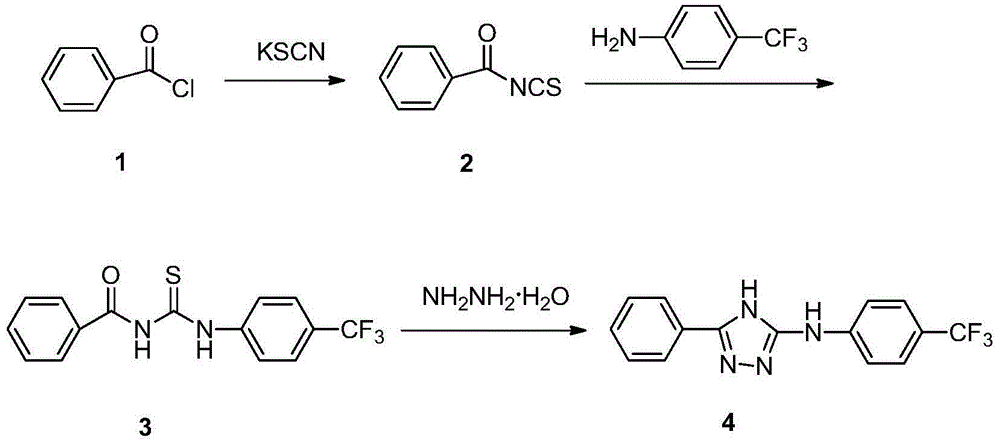
[0017] Preparation of intermediate benzoyl isothiocyanate (3)
[0018] Add 2.10 g (21.6 mmol) of potassium thiocyanate into a 100 ml three-necked flask, and add 60 ml of acetone to dissolve. Raise the temperature to 45-50°C, slowly add dropwise a mixed solution of 2.1ml (18mmol) of benzoyl chloride and 20ml of acetone, and continue the reaction for 3-4h after the dropwise addition. Let cool to room temperature. Suction filtration, washing with acetone, the collected filtrate can be directly used in the next step. Dissolve 2.9g (18mmol) of p-trifluoromethylaniline in 20ml of acetone, and add it dropwise to benzoyl isothiocyanate (2) within 30min, and heat up to reflux for 8-9h. TLC tracking. After the reaction was completed, the solvent was evaporated to dryness, diluted with ice water, stirred evenly, and the solid was collected by suction filtration, and recrystallized with ethanol to obtain 1-benzoyl-3-(4-trifluoromethylphenyl)thiourea (3), white Needle cryst...
Embodiment 2
[0022] Preparation of intermediate benzoyl isothiocyanate (3)
[0023] Add 2.10 g (21.6 mmol) of potassium thiocyanate into a 100 ml three-necked flask, and add 60 ml of acetone to dissolve. Raise the temperature to 45-50°C, slowly add dropwise a mixed solution of 2.1ml (18mmol) of benzoyl chloride and 20ml of acetone, and continue the reaction for 3-4h after the dropwise addition. Let cool to room temperature. Suction filtration, washing with acetone, the collected filtrate can be directly used in the next step. 6.0g (36mmol) of p-trifluoromethylaniline was dissolved in 20ml of acetone, and was added dropwise to benzoyl isothiocyanate (2) within 30min, and the temperature was raised to reflux for 12h. TLC tracking. After the reaction was completed, the solvent was evaporated to dryness, diluted with ice water, stirred evenly, and the solid was collected by suction filtration, and recrystallized with ethanol to obtain 1-benzoyl-3-(4-trifluoromethylphenyl)thiourea (3), white...
Embodiment 3
[0027] The preparation of intermediate benzoyl isothiocyanate (3) is the same as in Example 1.
[0028] Add 3.0 g (9 mmol) of 1-benzoyl-3-(4-trifluoromethylphenyl)thiourea (3), 50 ml of ethanol and 5 ml of acetic anhydride into a 100 ml three-necked flask. Heat to completely dissolve the solid, and add 80% hydrazine hydrate (46 mmol) in ethanol dropwise to the above solution. Reflux for 10 hours. After the reaction was completed, a white solid was precipitated after standing for cooling. Suction filtration, the collected solid was recrystallized with absolute ethanol to obtain 0.8 g of the target compound 3-phenyl-5-(4-trifluoromethylanilino)-4H-1,2,4-triazole (4). rate 29%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com