Preparation method of teriflunomide and intermediate thereof
A technology for teriflunomide and intermediates, which is applied in the field of preparation of teriflunomide and its intermediates, can solve the problems of corroded equipment, low total yield, cumbersome steps, etc., achieves improved yield, and is conducive to industrial production , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Embodiment 1 has the preparation of the compound of structure shown in formula II
[0118] In a 3L round-bottomed flask, 205g (3.15mol) of sodium azide, 400g (2.1mol, purchased from Aladdin), 52.4g (0.2mol, purchased from Aladdin), N-chlorosuccinimide 28g (0.2mol, purchased from Aladdin), tetrahydrofuran 2.4L, stirred and reacted at 15°C for 5 hours, followed the reaction until the reaction of the raw materials was completed. After the reaction was completed, 1.8 L of solvent was removed under reduced pressure, and then the reaction solution was poured into 2 L of distilled water, stirred for 30 min, filtered, and the filter cake was washed with 200 mL of distilled water and 200 mL of ethanol to obtain 388 g of off-white solid compound, the reaction weight yield was 86%.
[0119] The obtained off-white solid compound is identified by mass spectrometry, hydrogen nuclear magnetic resonance spectrum and carbon nuclear magnetic resonance spectrum, and its mass spectrum data...
Embodiment 2
[0121] Embodiment 2 has the preparation of the compound of structure shown in formula III
[0122] Transfer 388g (1.8mol) of the off-white solid compound obtained in Example 1 to a 3L round-bottomed flask, add 2.4L of toluene, heat and reflux at 110°C for 16 hours, follow the reaction and distill off the solvent after the reaction of the raw materials is complete, to obtain The crude product was washed with 300 mL of anhydrous ether and dried to obtain 354 g of light yellow solid compound.
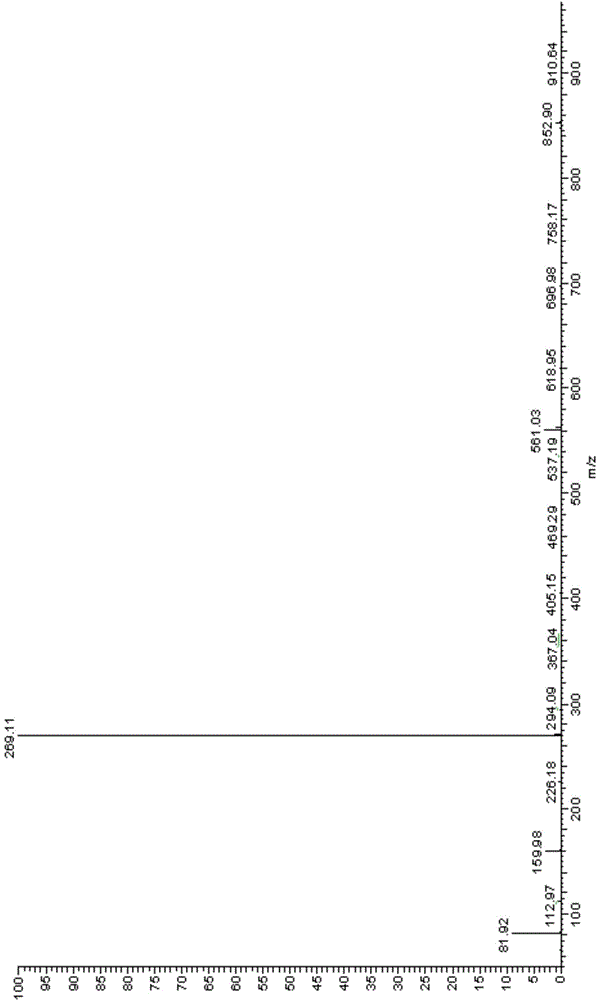
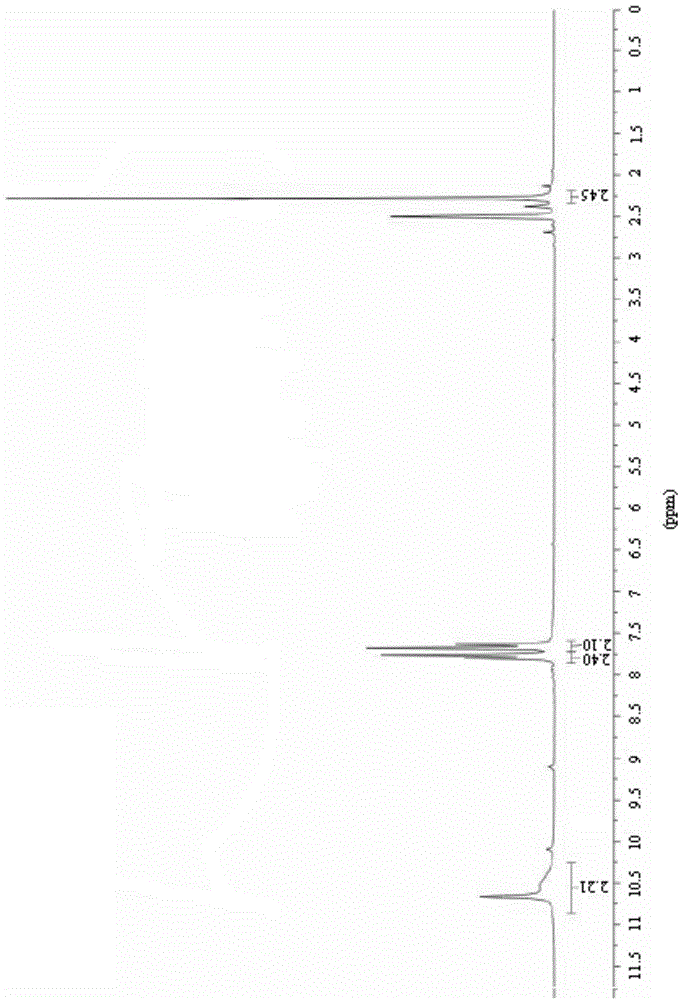
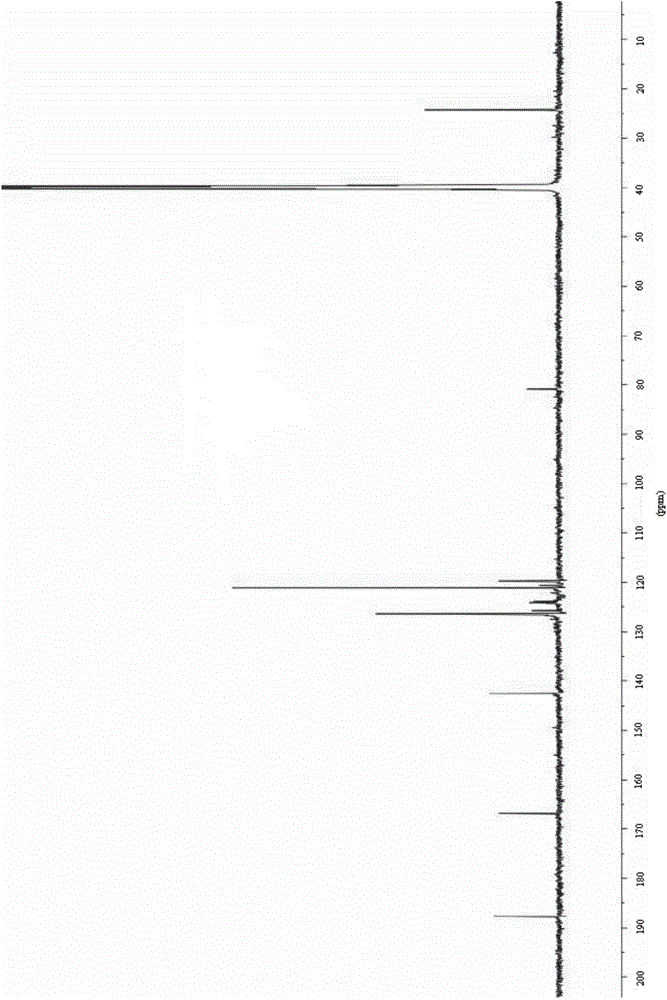
[0123] The obtained light yellow solid compound was identified by mass spectrometry, proton nuclear magnetic resonance and proton nuclear magnetic resonance. The mass spectrum data of the compound is [M+H + ]=188.3, its H NMR spectrum is: 1 H-NMR (DMSO-d6) δ: 7.41 (d, J=8.0Hz, 2H), 7.48 (d, J=8.0Hz, 2H), the carbon nuclear magnetic resonance spectrum is: 13 C-NMR (DMSO-d6) δ: 121.2, 121.9, 122.6, 123.3, 125.0, 126.8, 127.6, 133.3, 135.2ppm, so the compound prepared by the preparation ...
Embodiment 3
[0125] Example 3 Preparation of Teriflunomide
[0126] 354 g (1.8 mol) of the light yellow solid compound obtained in Example 2 was directly used in the synthesis of teriflunomide. Add 150 g (1.8 mol, purchased from Beijing Huawei Raycus Co., Ltd.), dry THF (1.5 L), and 80 g (2 mol, g / mL) of a compound having the structure shown in Formula IV into a 3 L round bottom flask. , stored in kerosene at a mass volume ratio of 60%), stirred at 15°C for 1 hour, then slowly added dropwise 800 mL of THF solution of 354 g (1.8 mol) of the light yellow compound prepared in Example 2, and the dropwise addition was completed in 1 hour, and then After the addition was complete, the reaction solution was heated to reflux, and reacted at 65° C. for 40 hours, with nitrogen protection during the reaction. Add 500mL of ice water after completion of the reaction to quench the reaction, adjust the pH of the reaction solution to neutral with 2mol / L of HCl, extract 3 times with EtOAc, and each dosage...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com