A kind of teriflunomide microemulsion, preparation method and application
A technology of teriflunomide and microemulsion, which is applied in the field of new dosage forms of teriflunomide, can solve the problems of long half-life and irritation, and achieve the effects of reducing fluctuations in the body, promoting absorption, and facilitating penetration and absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This example includes the following components:
[0034] Teriflunomide: 5 mg, isopropyl myristate: 2.106 g, soybean lecithin: 1.053 g, absolute ethanol: 1.053 g, deionized water: 0.783 g.
[0035] The preparation process is as follows:
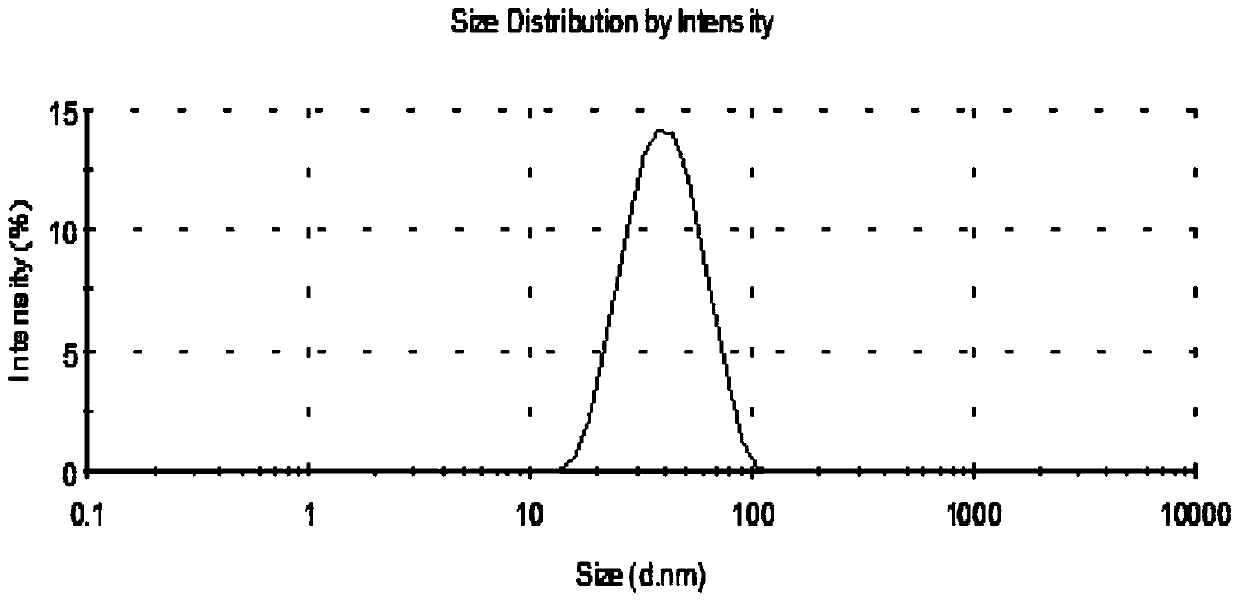
[0036] Accurately weigh IPM, soybean lecithin, and absolute ethanol. Mix and dissolve soybean lecithin and absolute ethanol first, then add IPM and mix thoroughly. Add teriflunomide reference substance into deionized water, ultrasonically dissolve, and slowly drop teriflunomide aqueous solution into the above liquid under stirring with a magnetic stirrer, and stir for 30 minutes to obtain a transparent mass ratio of 0.1% teriflunomide milk samples. The particle size distribution of teriflunomide microemulsion is detected by dynamic light scattering method, such as figure 1 As shown, the microemulsion particle size ranges from 10 to 100nm, the particle size is uniform, and the appearance is clear, such as figure 2 (a) shown.
Embodiment 2
[0038] This example includes the following components:
[0039] Teriflunomide: 12.5mg, isopropyl myristate: 2.106g, soybean lecithin: 1.053g, absolute ethanol: 1.053g, deionized water: 0.783g.
[0040] The preparation process is as follows:
[0041]Accurately weigh IPM, soybean lecithin, and absolute ethanol. Mix and dissolve soybean lecithin and absolute ethanol first, then add IPM and mix thoroughly. Add the teriflunomide reference substance into deionized water, dissolve it ultrasonically, slowly drop the teriflunomide aqueous solution into the above liquid under stirring with a magnetic stirrer, and stir for 30 minutes to obtain a transparent 0.25% teriflunomide microemulsion sample . The particle size distribution of the teriflunomide microemulsion was detected by the dynamic light scattering method. The particle size range of the microemulsion was 10-100nm, the particle size was uniform, and the appearance was clear. The results were as follows: figure 2 (b) shown. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com