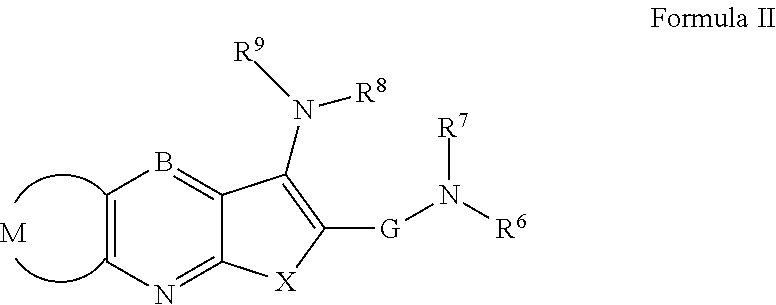
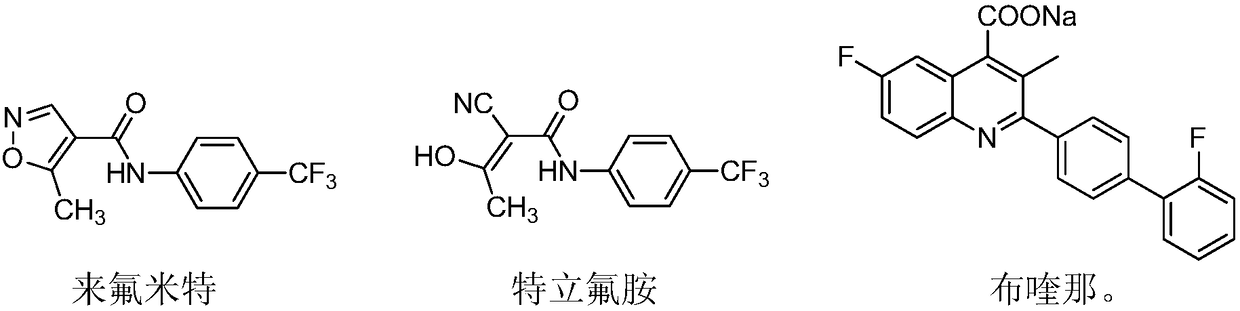
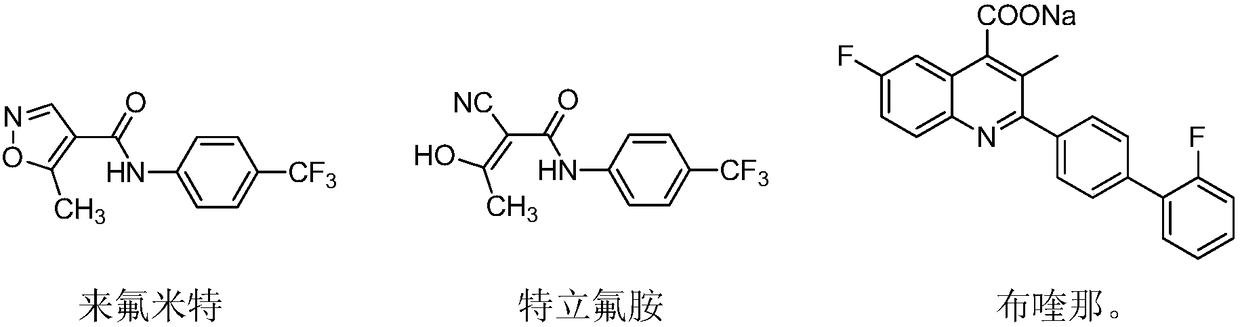
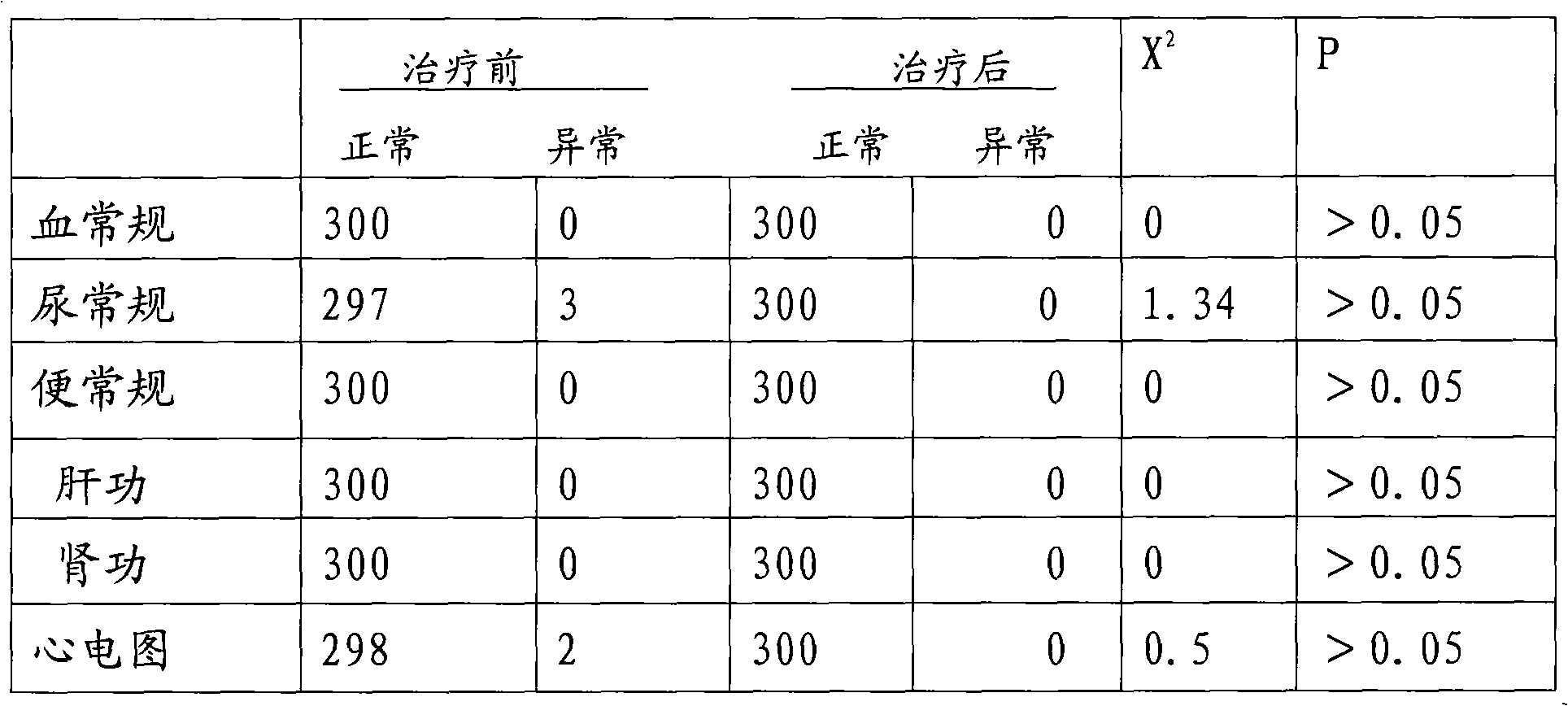
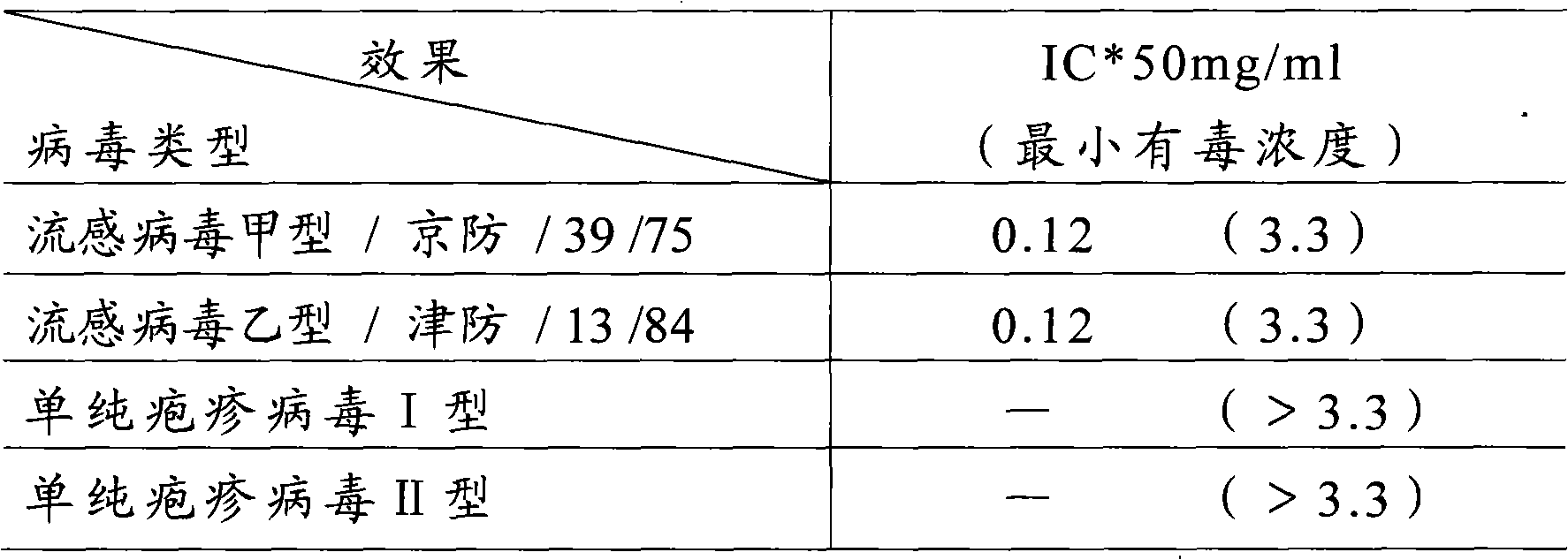
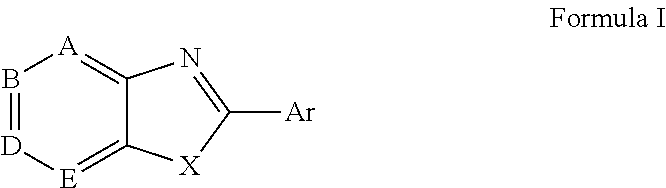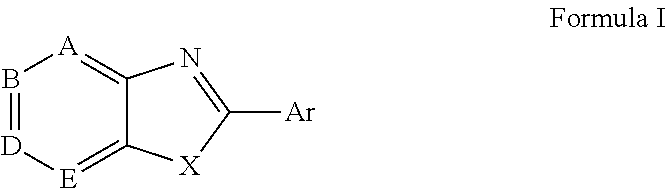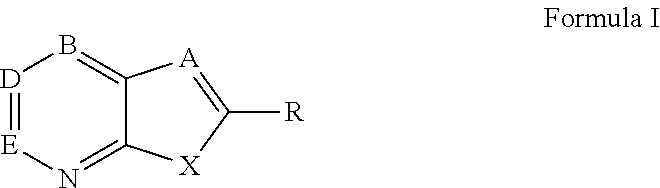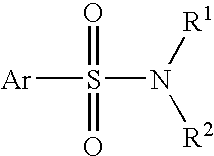Patents
Literature
245 results about "Japanese encephalitis viruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Japanese encephalitis —previously known as Japanese B encephalitis to distinguish it from von Economo's A encephalitis—is a disease caused by the mosquito-borne Japanese encephalitis virus. The Japanese encephalitis virus is a virus from the family Flaviviridae.
Chimeric and/or growth-restricted flaviviruses
InactiveUS6184024B2BiocideSsRNA viruses positive-senseViral matrix proteinJapanese encephalitis viruses
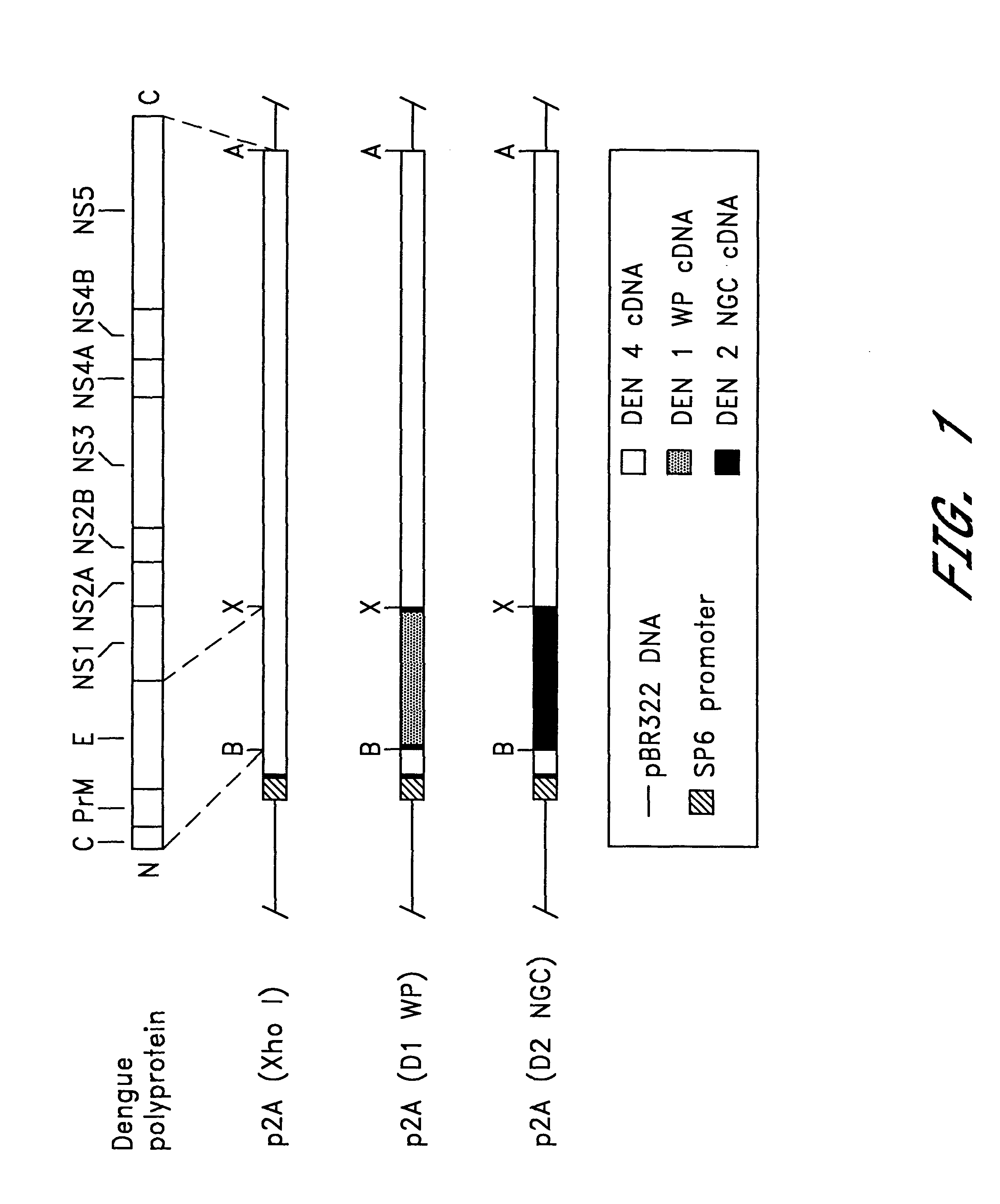
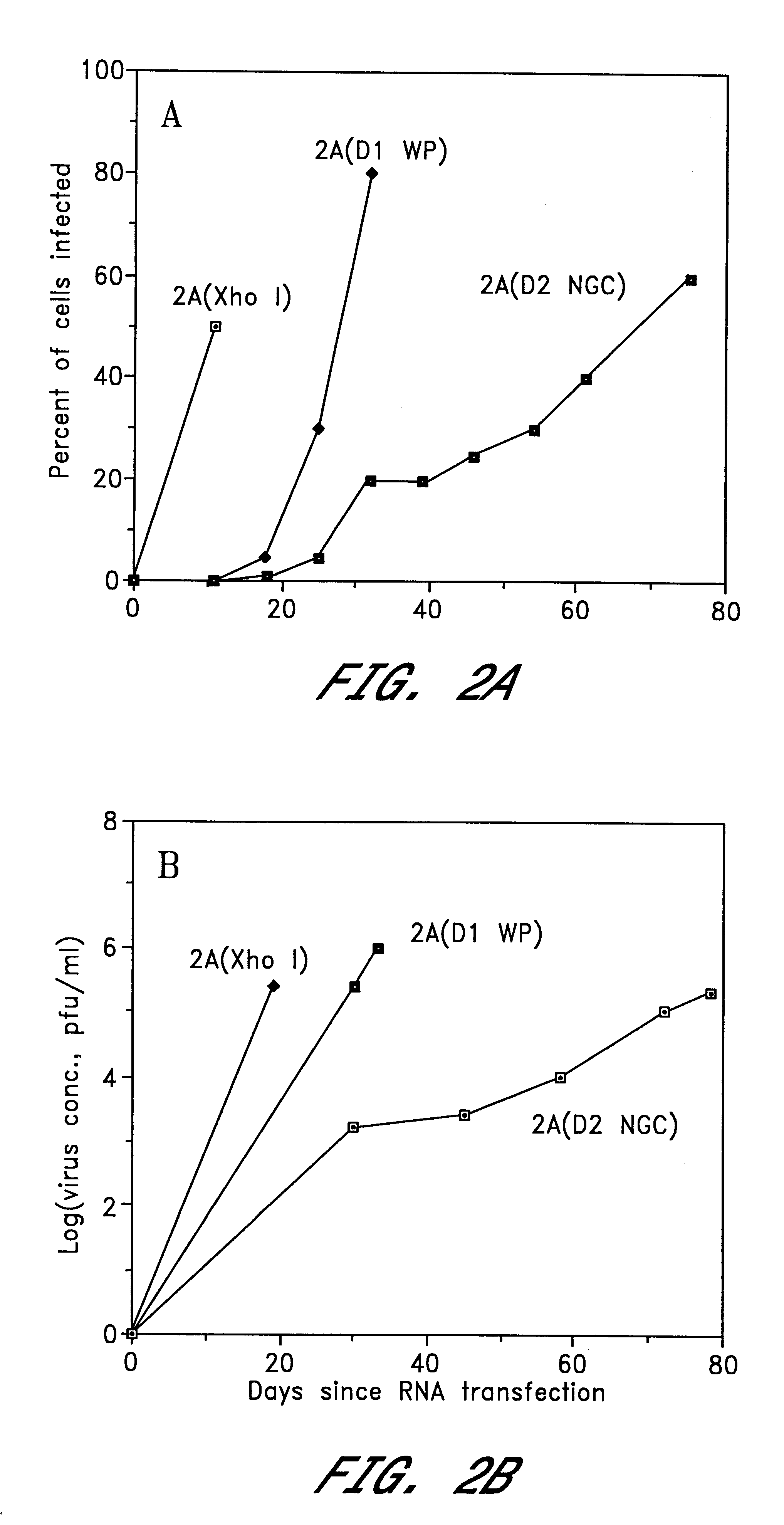
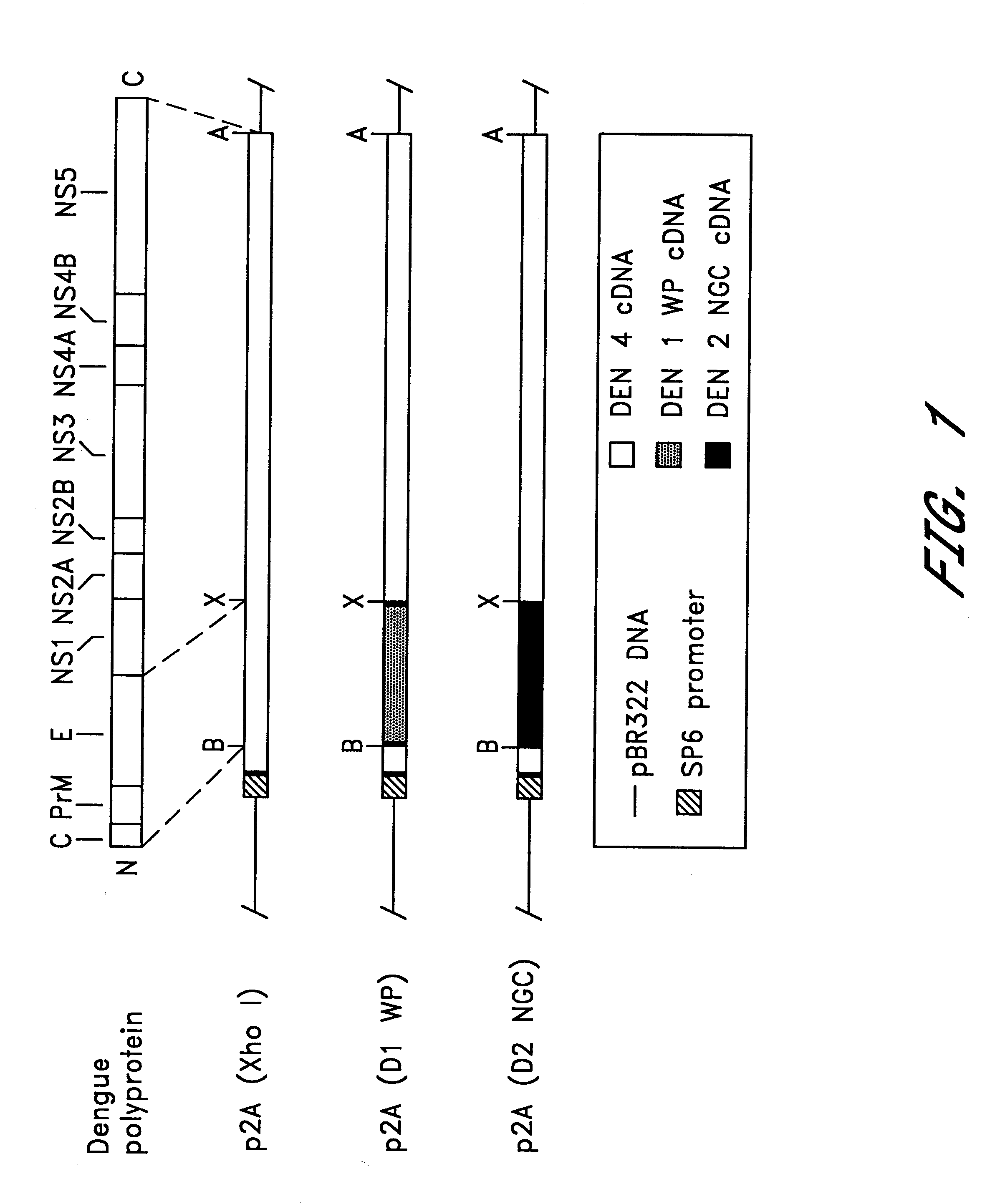
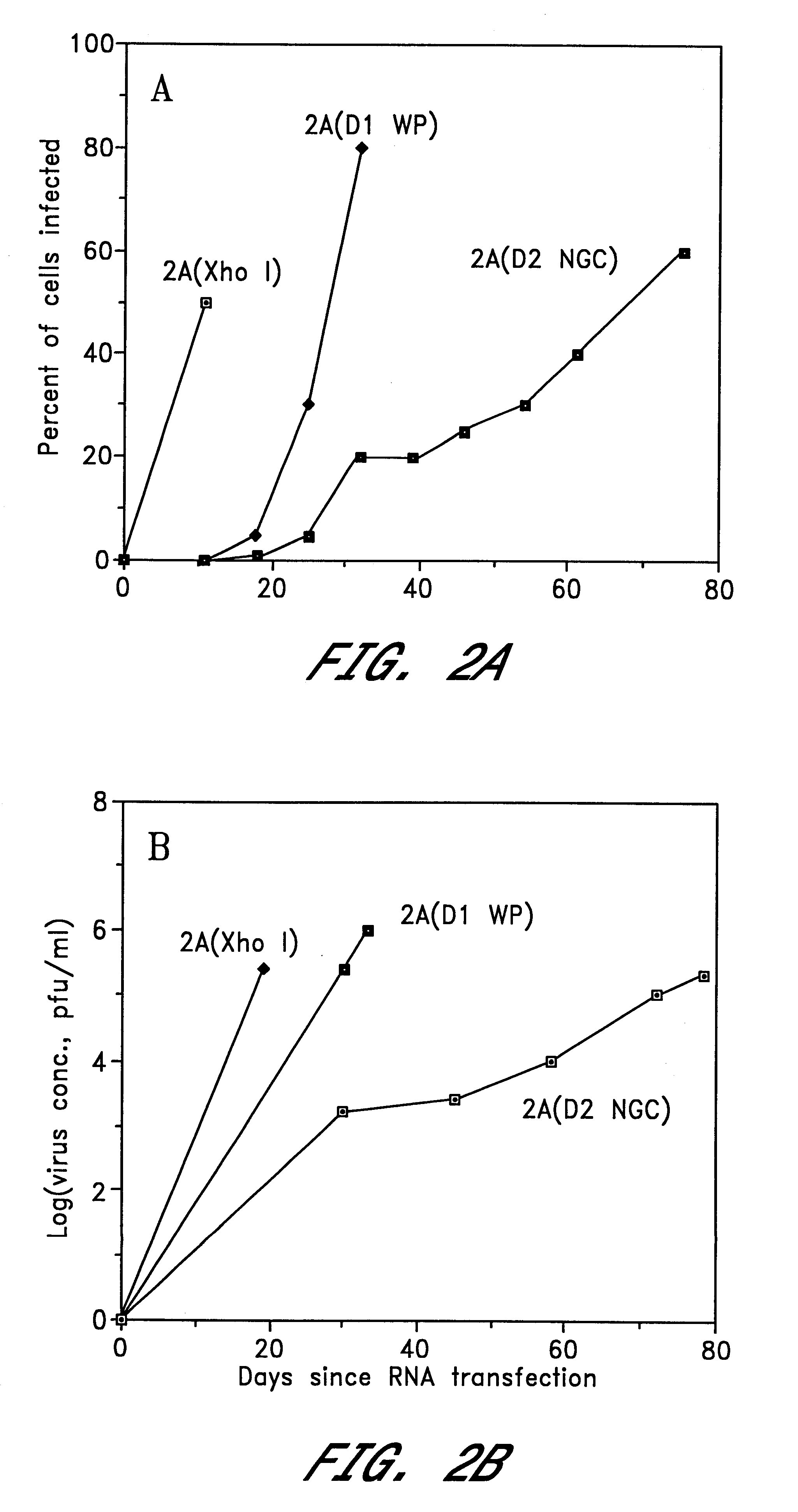
The invention includes a chimeric virus for use in a vaccine preparation having a genome comprising nucleic acid sequences encoding at least one structural protein from one flavivirus and nucleic acid sequences encoding nonstructural protein from another flavivirus. The genome preferably includes mutations within the viral genome that reduce virus virulence and in a particularly preferred embodiment these vaccines are directed to flaviviruses such as dengue virus, tick-borne encephalitis virus and Japanese encephalitis virus. The invention also includes a baculovirus having a recombinant dengue cDNA sequence which encodes: (1) dengue virus capsid protein, pre-matrix protein, envelope glycoprotein and NS1 and NS2a nonstructural proteins or (2) dengue envelope glycoprotein or (3) dengue non-structural proteins NS1 and NS2a. The invention further includes a baculovirus having a recombinant Japanese B encephalitis virus cDNA sequence which encodes the Japanese B encephalitis virus capsid protein, pre-matrix protein, envelope glycoprotein and non-structural proteins NS1 and NS2a. The invention further includes a vaccine and a method to produce that vaccine.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Diagnostic test for West Nile virus
InactiveUS20040197769A1More sensitiveEasy to useViral antigen ingredientsMicrobiological testing/measurementSt Louis encephalitis virusSerum ige
The present invention provides a rapid and sensitive method for the detection of a West Nile virus (WNV), Japanese encephalitis virus (JEV), St. Louis encephalitis virus (SLEV) and Dengue virus (DENV) and antibodies directed against thereof involving contacting a biological specimen suspected of being infected with WNV, JE, SLE or DEN with a substantially purified and isolated WNV E glycoprotein or subfragment thereof having a native conformation wherein the E glycoprotein or subfragment thereof has a reactivity with antibodies against WNV and a cross-reactivity with antibodies against JEV, SLEV and DENV. The instant invention further provides a rapid, sensitive, and consistent method for the specific detection of WNV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-WNV antibodies but not cross-reactive with antibodies against other flaviviruses such as JEV, SLEV, or DENV. The present invention also provides a rapid, sensitive, and consistent method for the specific detection of DENV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-DENV antibodies but do not cross-react with antibodies against other flaviviruses such as JEV, SLEV, or WNV. Further, the DENV NS5 antigens are serospecific and do not cross react with antibodies to other DENV strains. Thus, the method of the present invention provides a manner by which to discriminate infections by each DENV strain. Further, diagnostic kits for carrying out the methods are provided. The methods and kits for carrying out the methods of the invention are rapid and require as little as 10 minutes to detect a result.
Owner:HEALTH RES INC
Treatment and prevention of dengue virus infections
Methods and pharmaceutical compositions for treating viral infections, by administering certain 2-aryl-benzothiazole or 2-heteroaryl-benzothiazole derivative compounds in therapeutically effective amounts are disclosed. Methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment and prophylaxis of viral infections such as caused by flavivirus is disclosed, i.e., including but not limited to, Dengue virus, West Nile virus, yellow fever virus, Japanese encephalitis virus, and tick-borne encephalitis virus.
Owner:SIGA TECH INC
Chimeric and/or growth-restricted flaviviruses
InactiveUS6676936B1BiocideSsRNA viruses positive-senseVirulent characteristicsJapanese B Encephalitis Virus
The invention includes a chimeric virus for use in a vaccine preparation having a genome comprising nucleic acid sequences encoding at least one structural protein from one flavivirus and nucleic acid sequences encoding nonstructural protein from another flavivirus. The genome preferably includes mutations within the viral genome that reduce virus virulence and in a particularly preferred embodiment these vaccines are directed to flaviviruses such as dengue virus, tick-borne encephalitis virus and Japanese encephalitis virus. The invention also includes a baculovirus having a recombinant dengue cDNA sequence which encodes: (1) dengue virus capsid protein, pre-matrix protein, envelope glycoprotein and NS1 and NS2a nonstructural proteins or (2) dengue envelope glycoprotein or (3) dengue non-structural proteins NS1 and NS2a. The invention further includes a baculovirus having a recombinant Japanese B encephalitis virus cDNA sequence which encodes the Japanese B encephalitis virus capsid protein, pre-matrix protein, envelope glycoprotein and non-structural proteins NS1 and NS2a. The invention further includes a vaccine and a method to produce that vaccine.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Diagnostic test for west nile virus
ActiveUS20060115896A1More sensitiveEasy to useAnimal cellsMicrobiological testing/measurementDiagnostic testFlavivirus
The present invention provides a rapid and sensitive method for the detection of a West Nile virus (WNV), Japanese encephalitis virus (JEV), St. Louis encephalitis virus (SLEV) and Dengue virus (DENV) and antibodies directed against thereof involving contacting a biological specimen suspected of being infected with WNV, JE, SLE or DEN with a substantially purified and isolated WNV E glycoprotein or subfragment thereof having a native conformation wherein the E glycoprotein or subfragment thereof has a reactivity with antibodies against WNV and a cross-reactivity with antibodies against JEV, SLEV and DENV. The instant invention further provides a rapid, sensitive, and consistent method for the specific detection of WNV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-WNV antibodies but not cross-reactive with antibodies against other flaviviruses such as JEV, SLEV, or DENV. The present invention also provides a rapid, sensitive, and consistent method for the specific detection of DENV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-DENV antibodies but do not cross-react with antibodies against other flaviviruses such as JEV, SLEV, or WNV. Further, the DENV NS5 antigens are serospecific and do not cross react with antibodies to other DENV strains. Thus, the method of the present invention provides a manner by which to discriminate infections by each DENV strain. Further, diagnostic kits for carrying out the methods are provided. The methods and kits for carrying out the methods of the invention are rapid and require as little as 10 minutes to detect a result. The invention also provides monoclonal antibodies against WNV NS5 and DENV NS5 antigen and their use in detecting WNV and DENV infections in a biological sample.
Owner:HEALTH RES INC
Host targeted inhibitors of dengue virus and other viruses
ActiveUS20150166532A1Delay or minimize one or more symptoms associatedReduces and avoids symptom and causeBiocideOrganic chemistryHerpes simplex diseaseDisease injury
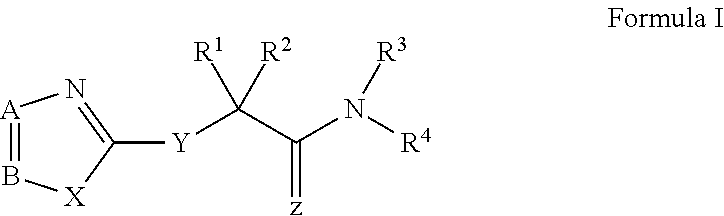
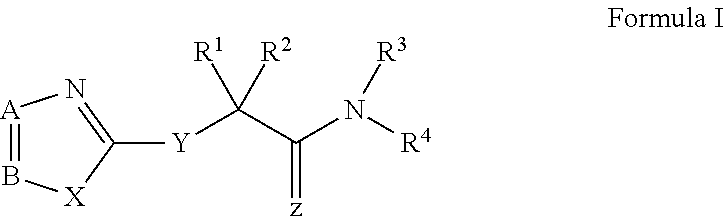
Novel antiviral compounds of Formulae (I)-(III) are provided: (I) (II) (III) The inventive compounds, pharmaceutical compositions thereof, and kits including the inventive compounds are useful for the prevention and treatment of infectious diseases caused by viruses, for example, by Flaviviridae virus (e.g., Dengue virus (DENY)), Kunjin virus, Japanese encephalitis virus, vesicular stomatitis virus (VSV), herpes simplex virus 1 (HSV-1), human cytomegalovirus (HCMV), poliovirus, Junin virus, Ebola virus, Marburg virus (MARV), Lassa fever virus (LASV), Venezuelan equine encephalitis virus (VEEV), or Rift Valley Fever virus (RVFV).
Owner:DANA FARBER CANCER INST INC +1
Vaccines Against Japanese Encephalitis Virus and West Nile Virus
ActiveUS20070269458A1Decrease viscerotropism/viremiaHigh genetic stabilitySsRNA viruses positive-senseSugar derivativesViral VaccineWest Nile virus RNA
The invention provides attenuated Flavivirus vaccines, such as vaccines against Japanese encephalitis virus and West Nile virus, as well as methods of making and using these vaccines.
Owner:SANOFI PASTEUR BIOLOGICS CO
Thienopyridine Derivatives for the Treatment and Prevention of Dengue Virus Infections
Methods and pharmaceutical compositions for treating viral infections, by administering certain thienopyridine derivative compounds in therapeutically effective amounts are disclosed. Methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment of viral infections such as caused by flavivirus is disclosed, i.e., including but not limited to, Dengue virus, West Nile virus, yellow fever virus, Japanese encephalitis virus, and tick-borne encephalitis virus.
Owner:SIGA TECH INC
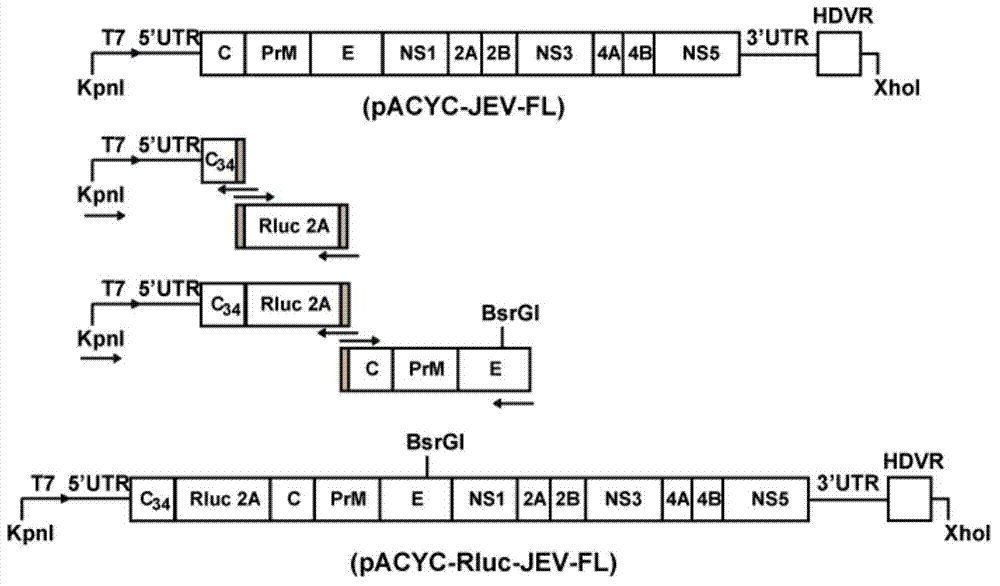
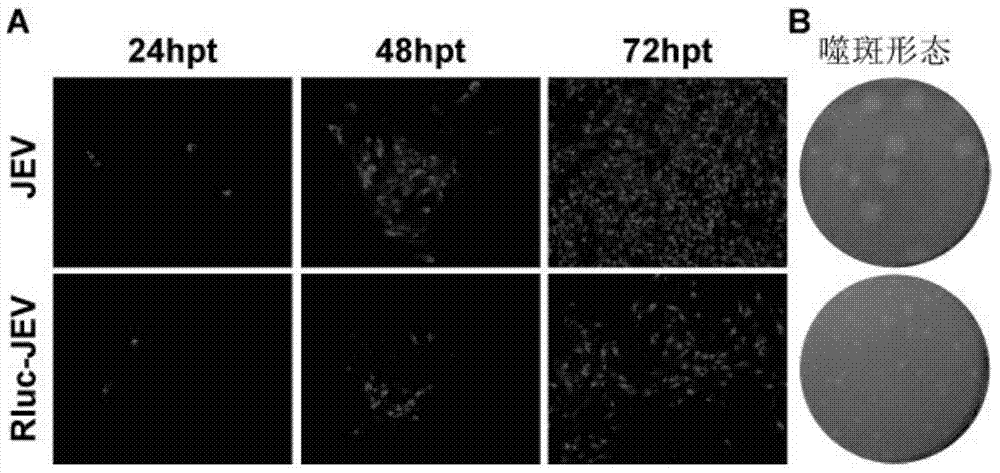
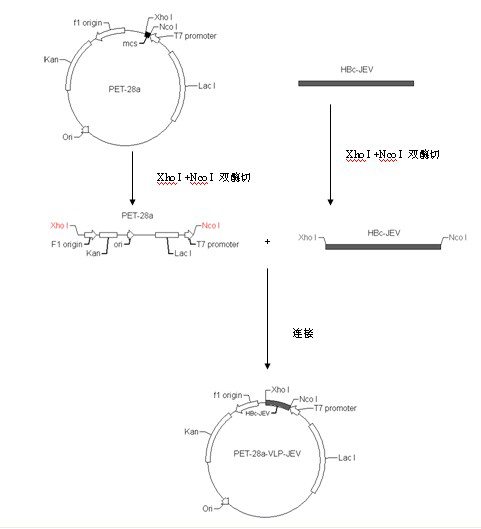
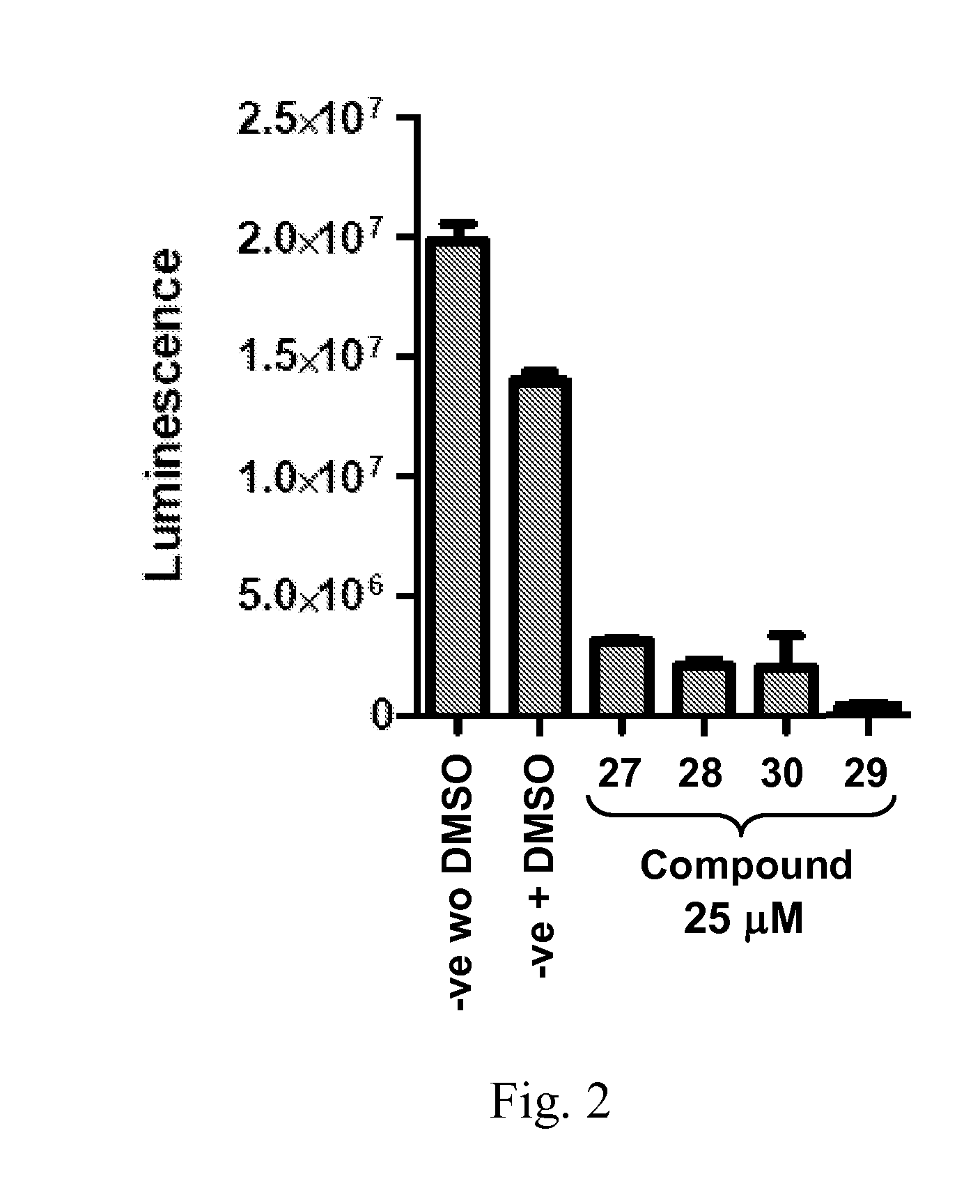
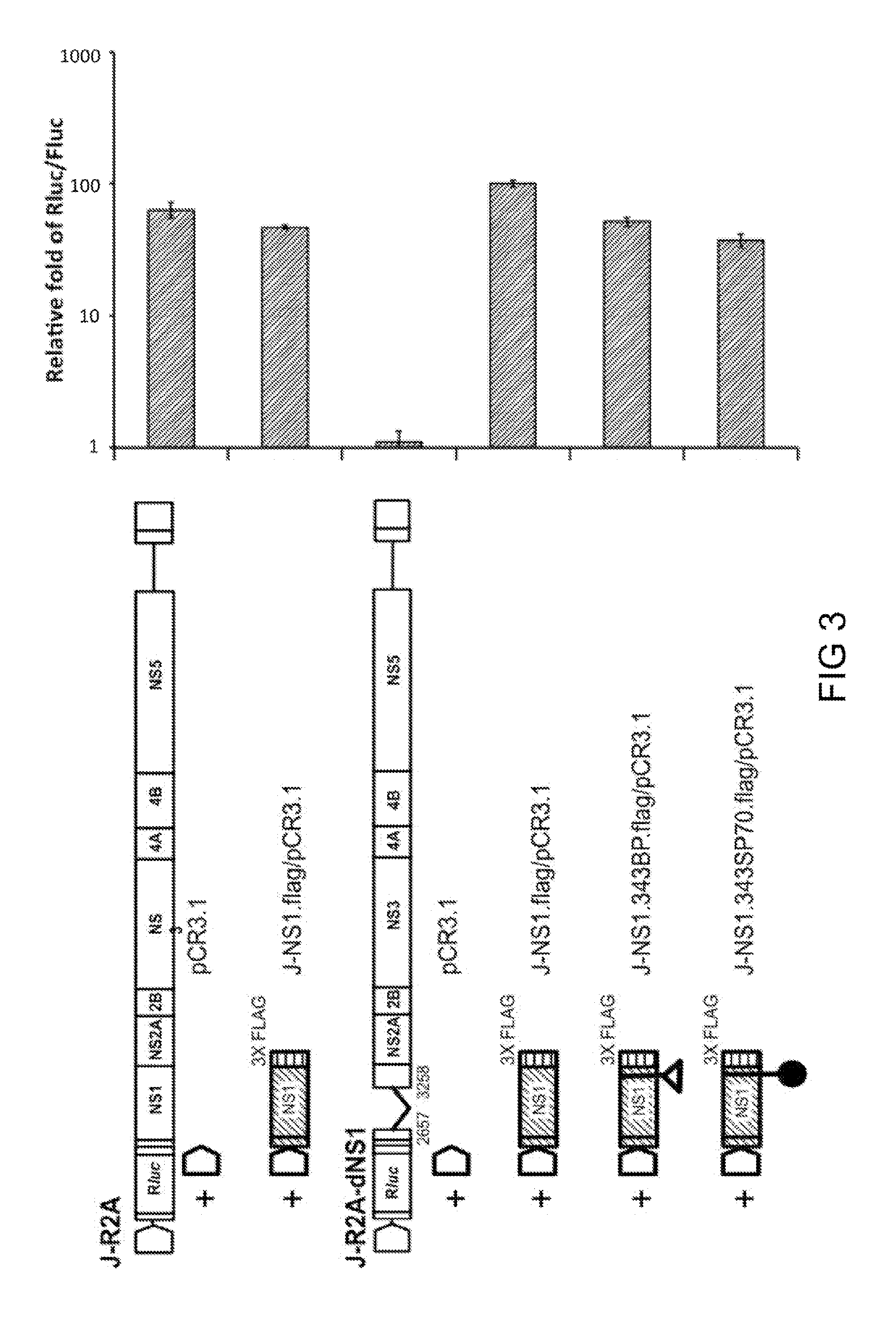
Japanese encephalitis virus (JEV) infectious clone with luciferase gene and building method and application thereof
InactiveCN103497972ASolve the phenomenon of not copyingFast growing trendMicrobiological testing/measurementFluorescence/phosphorescenceLuciferase GeneVaccine evaluation
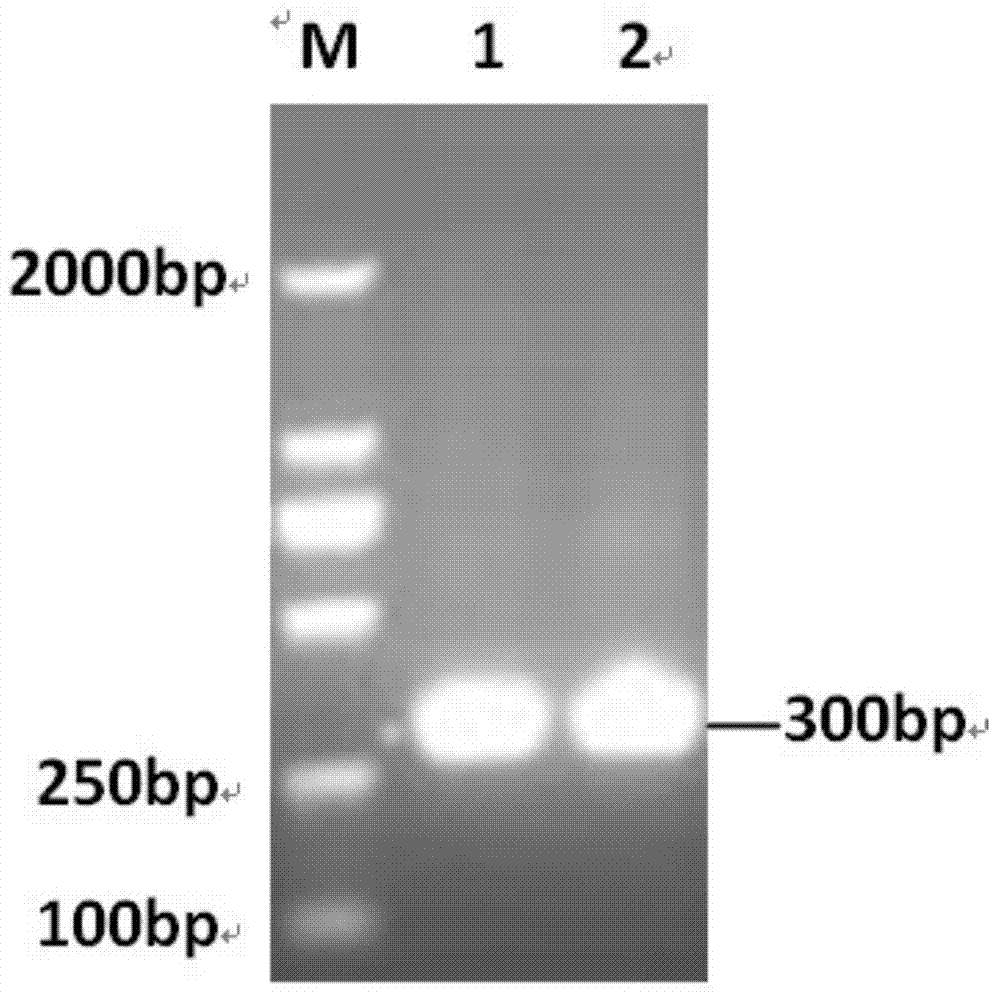
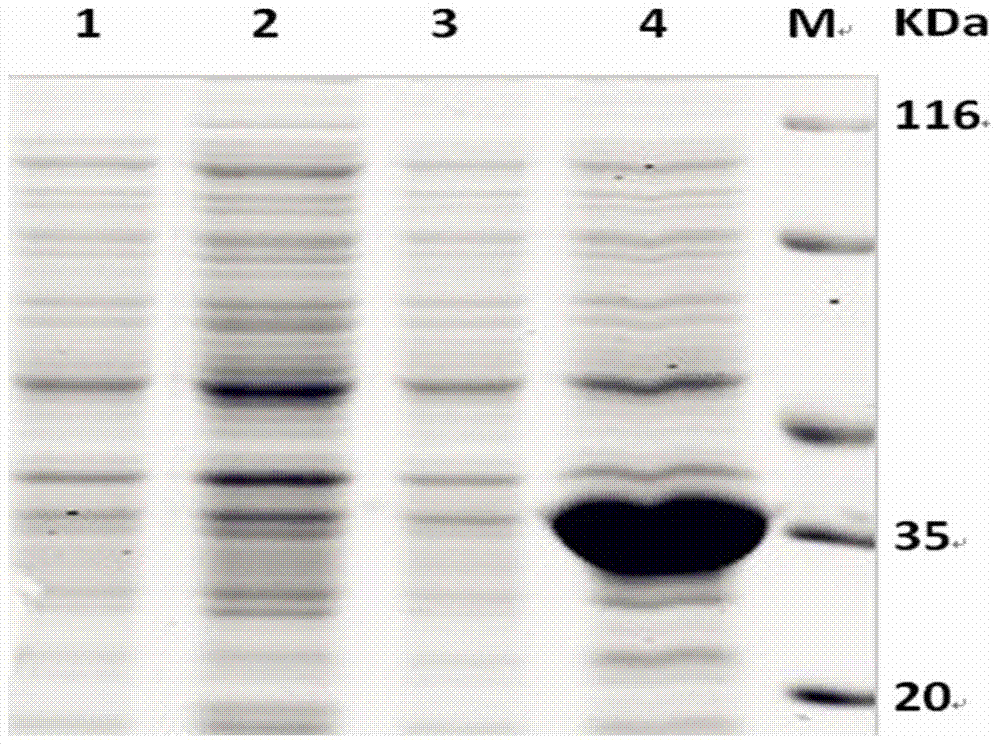
The invention discloses a Japanese encephalitis virus (JEV) infectious clone with a luciferase gene, and a building method and application thereof. The JEV infectious clone with the luciferase gene is prepared by the following steps: (1) sectional synthesis of a JEV SA14 strain gene sequence; (2) assembling and building of the JEV infectious clone; (3) building of the JEV infectious clone with the luciferase gene. It is proved that a Rluc-JEV report virus with the same growth tendency as the JEV virus can be saved by the JEV infectious clone with the luciferase gene built by the method, and the JEV infectious clone has wide application value in the aspects of an animal model, virus replication and pathogenesis, drug screening and drug action mechanism, live animal imaging and vaccine evaluation and the like through the experiments of immunofluorescence, Rluc activity detection, virus bacteriophage plaque, drug inhibition, live animal imaging, vaccine evaluation and the like.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Antiviral Drugs for Treatment or Prevention of Dengue Infection
Compounds, methods and pharmaceutical compositions for treating viral infections, by administering certain compounds in therapeutically effective amounts are disclosed. Methods for preparing the compounds and methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment and prophylaxis of viral infections such as caused by flavivirus is disclosed, i.e., including but not limited to, Dengue virus, West Nile virus, yellow fever virus, Japanese encephalitis virus, and tick-borne encephalitis virus.
Owner:SIGA TECH INC
Samll molecule inhibitors for the treatment or prevention of dengue virus infection
Methods and pharmaceutical compositions for treating viral infections, by administering certain compounds in therapeutically effective amounts are disclosed. Methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment and prophylaxis of viral infections such as caused by flavivirus is disclosed, i.e., including but not limited to, Dengue virus, West Nile virus, yellow fever virus, Japanese encephalitis virus, and tick-borne encephalitis virus.
Owner:SIGA TECH INC
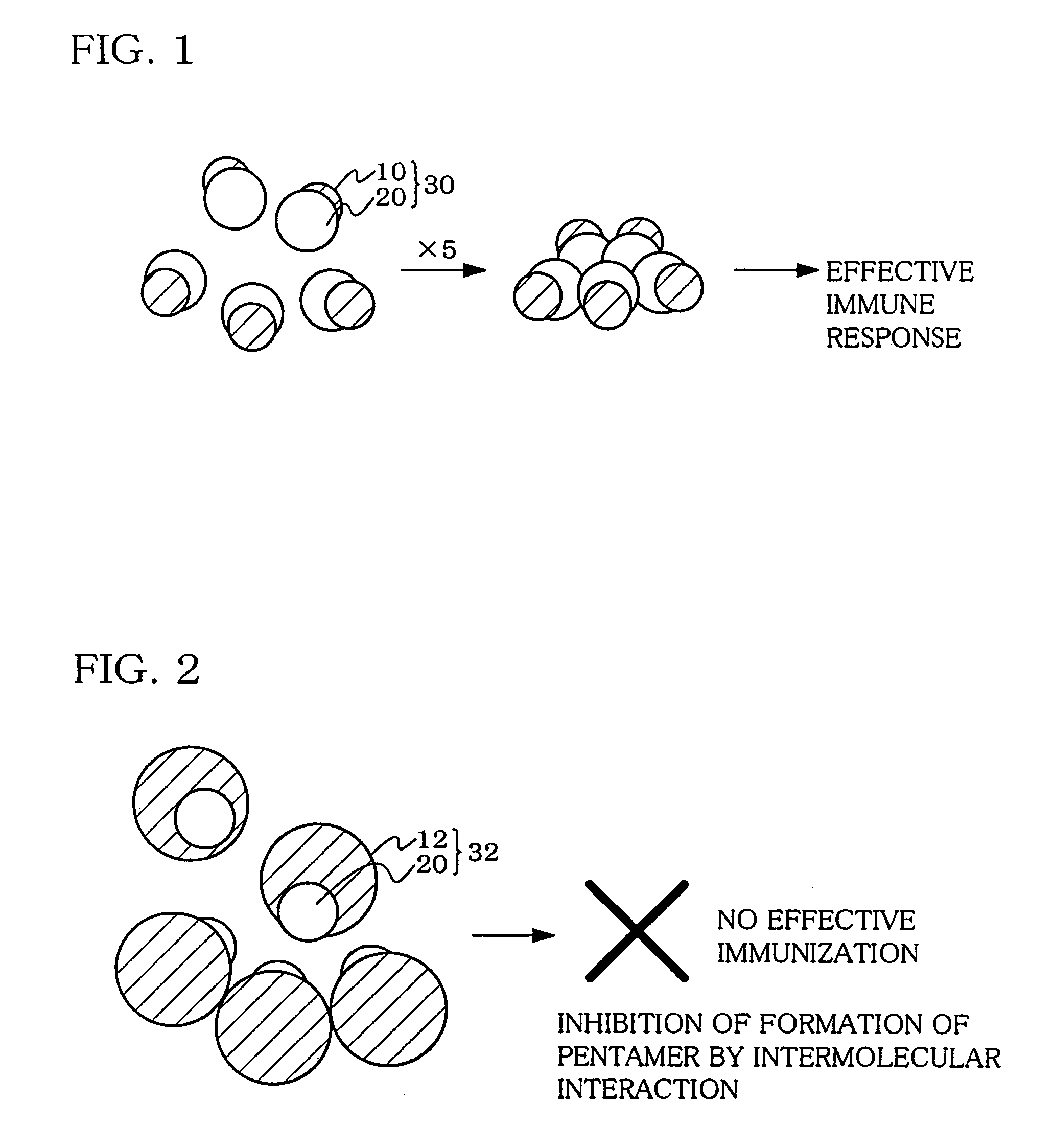
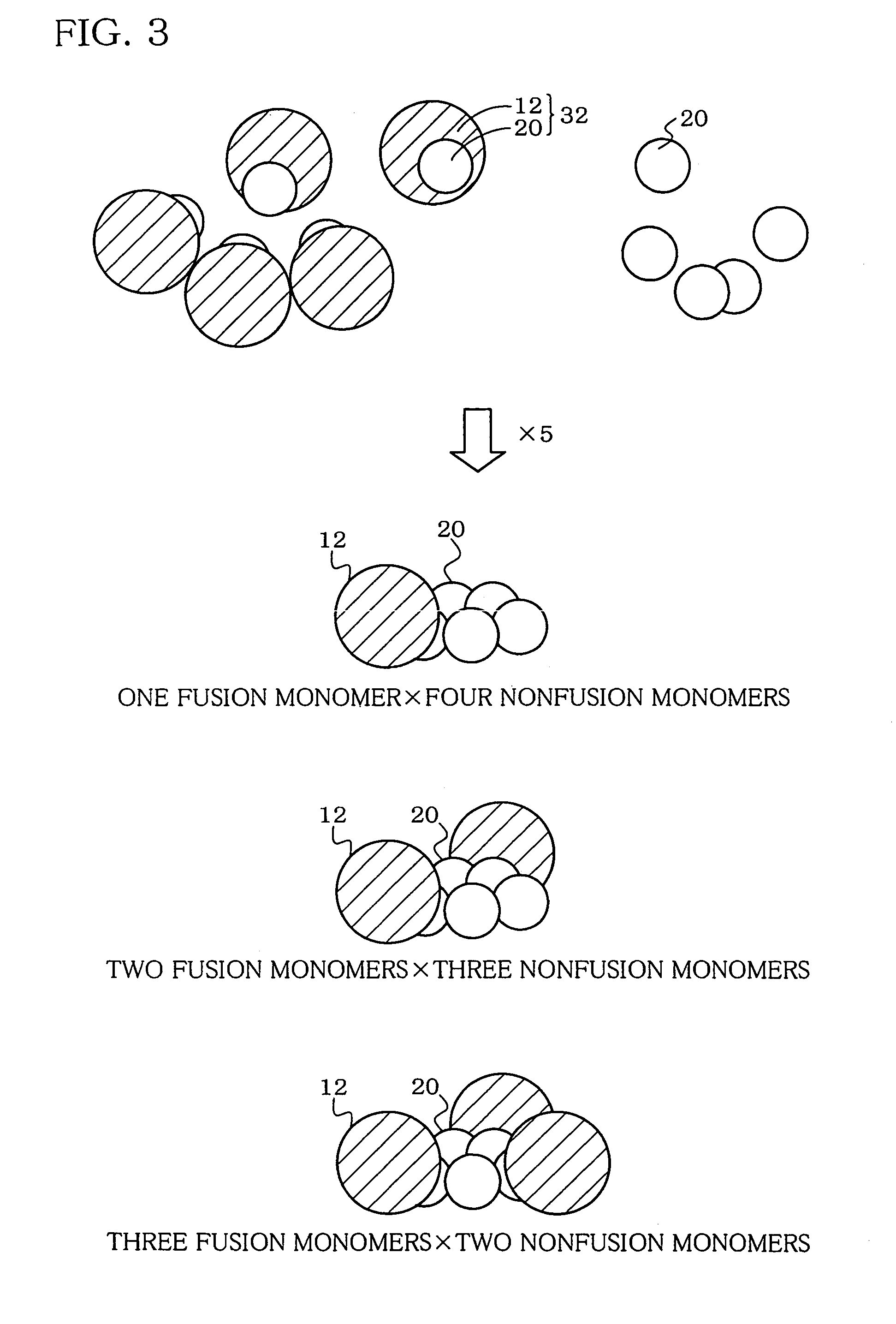
Hetero type pentamer recombinant vaccine
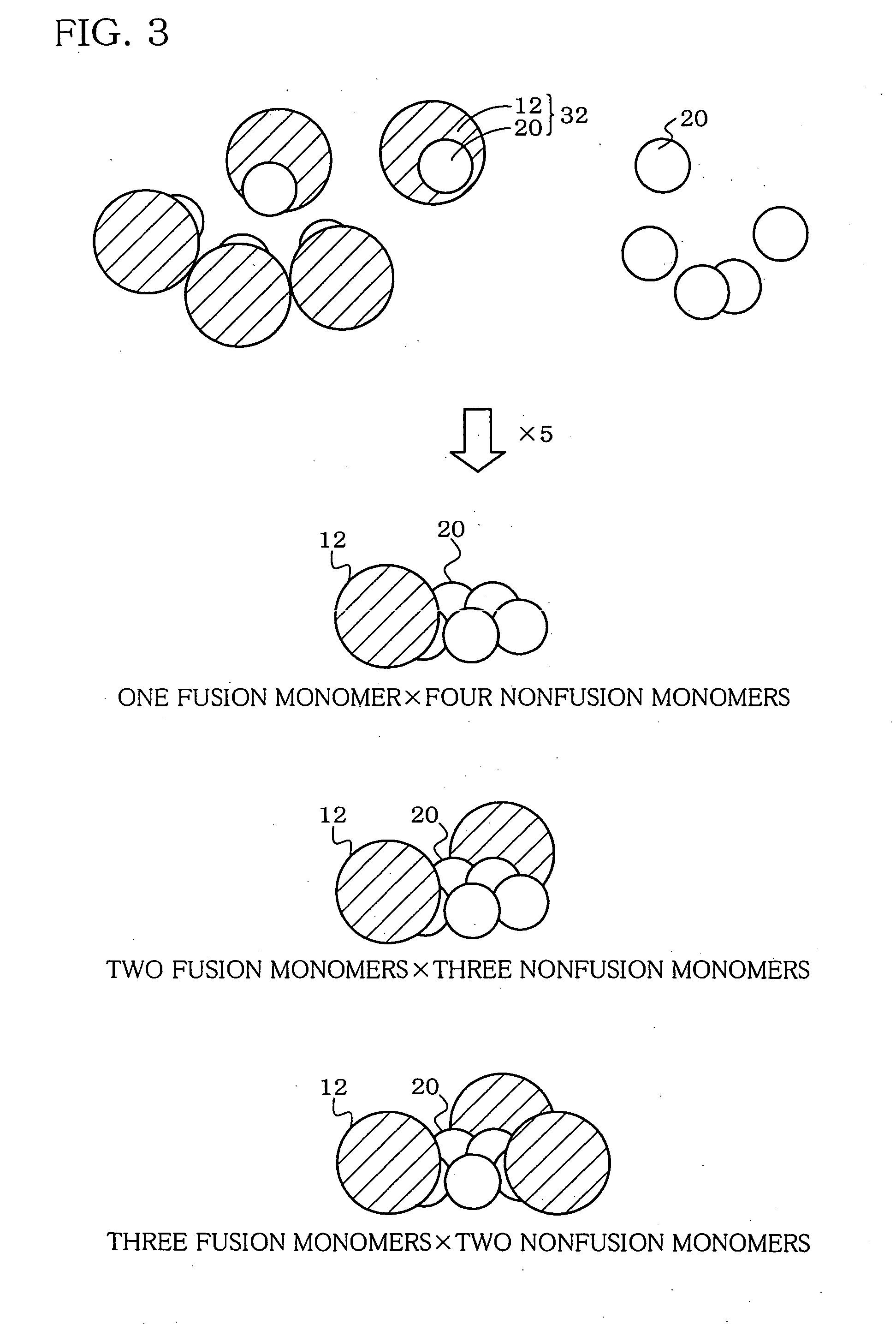
A heteropentamer composed of a fusion monomer (32) comprising a fusion protein of an antigenic amino acid sequence and an amino acid sequence of a monomer of a mucous membrane-binding protein and a nonfusion monomer (20) of an amino acid sequence of a monomer of a mucous membrane-binding protein. A homopentamer composed of a fusion monomer including a fusion protein of an antigen derived from an envelope protein of Japanese encephalitis virus and an amino acid sequence of a monomer of a mucous membrane-binding protein. These heteropentamer and the homopentamer can be used as a vaccine.
Owner:ADVANCED MEDICAL BIOLOGICAL SCI INST +1
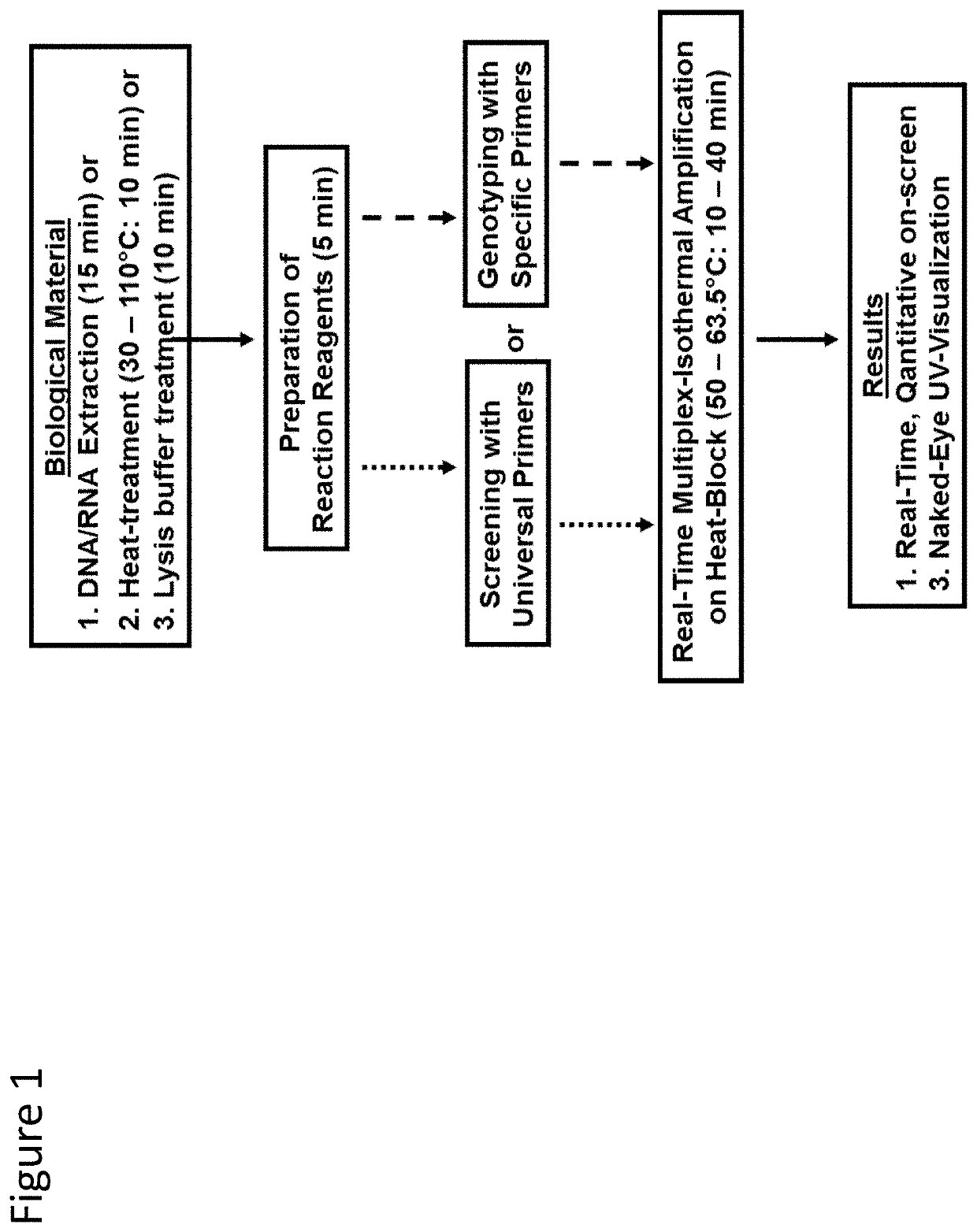
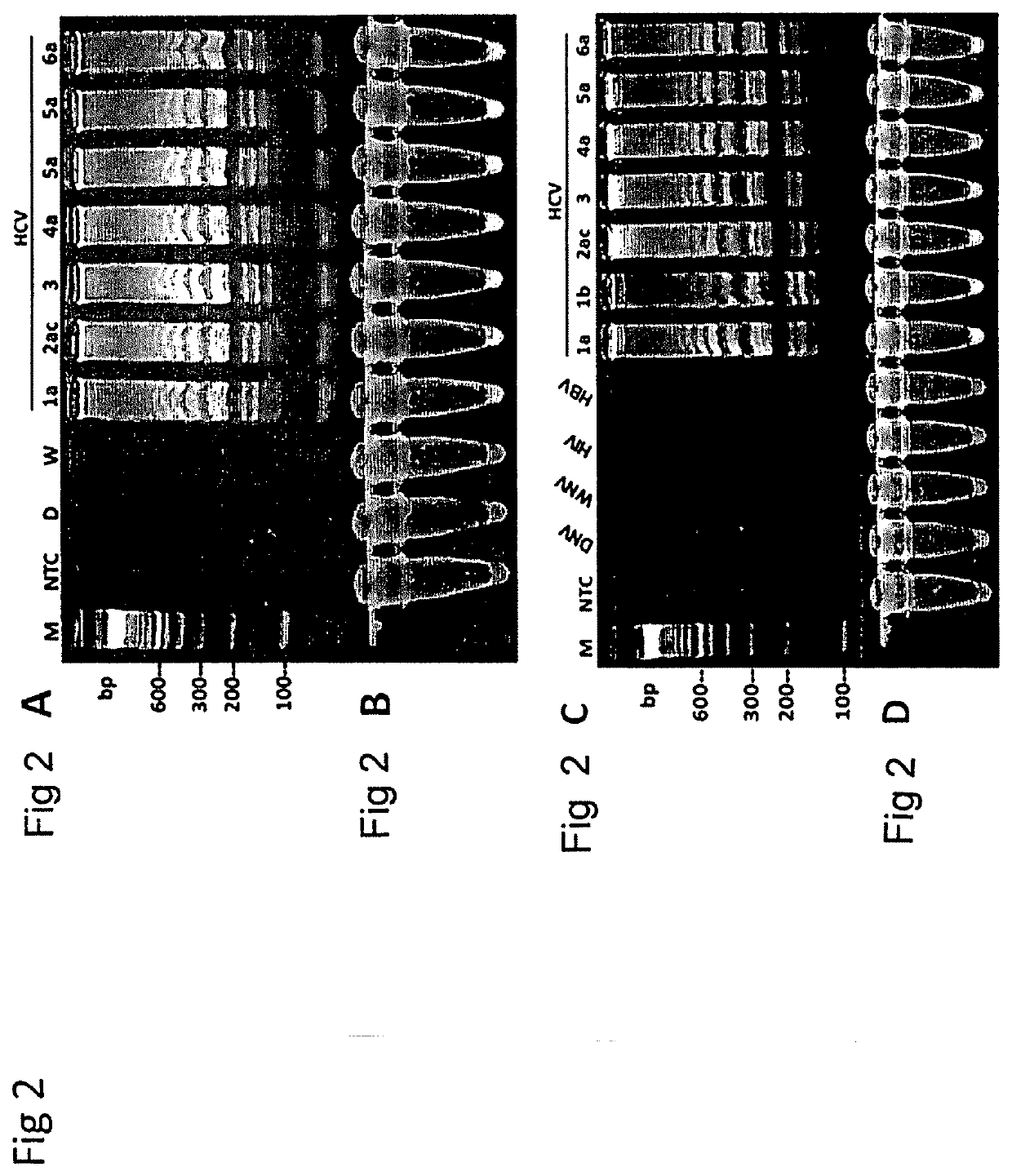
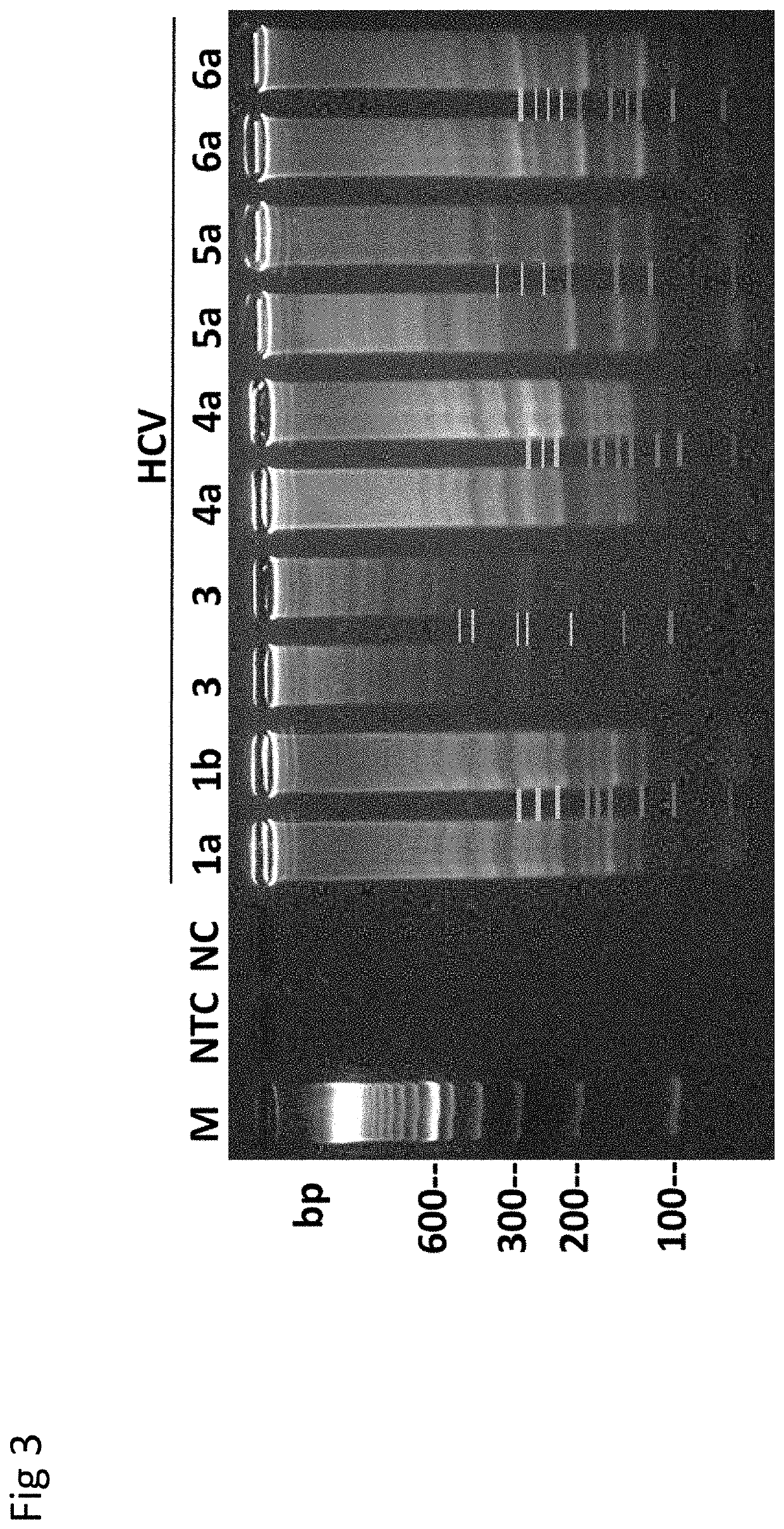
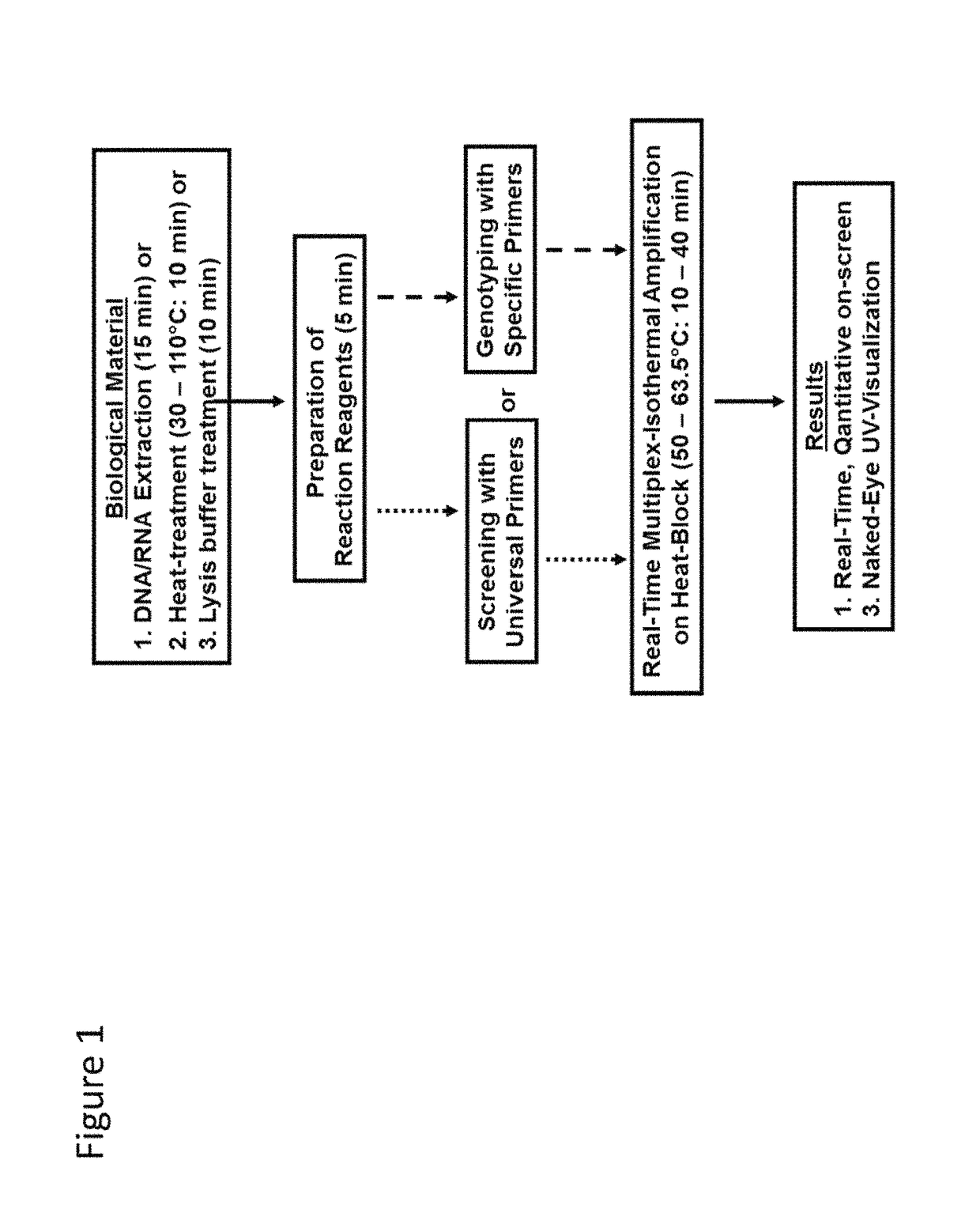
Methods for real-time multiplex isothermal detection and identification of bacterial, viral, and protozoan nucleic acids
ActiveUS20200048722A1Flexible processFacilitate strand displacementMicrobiological testing/measurementAgainst vector-borne diseasesProtozoaLoop-mediated isothermal amplification
Herein disclosed are rapid real-time isothermal multiplex methods of detecting, identifying and quantifying bacterial, viral, and protozoan nucleic acids in a sample. These include contacting the sample with two or more sets of pathogen-specific reverse transcription loop-mediated isothermal amplification primers and novel oligofluorophores specific for the target bacterial, viral, and parasitic nucleic acids of interest such as human immunodeficiency virus, Ebola virus, Marburg virus, Yellow fever virus, hepatitis-B virus, Lassa fever virus, Plasmodium, hepatitis-C virus, hepatitis-E virus, dengue virus, Chikungunya virus, Japanese Encephalitis virus, Middle Eastern Respiratory Syndrome Corona virus, Mycobacterium, West Nile virus, Cytomegalovirus, Parvovirus, Leishmania, Trypanosoma, and Zika virus nucleic acids, under conditions sufficient to produce detectable real-time amplification signals in about 10 to 40 minutes. The amplification signals are produced by pathogen-specific fluorogenic labels included in one or more of the primers. Also, novel reaction and sample lysis buffers, primers, and kits for rapid multiplex detection, quantification, and identification of bacterial, viral, and protozoan nucleic acids by real-time isothermal amplification are herein disclosed.
Owner:NYAN DOUGBEH CHRIS
Artificial cpg single-stranded oligodeoxynucleotide and antiviral use thereof
The present invention provides a series of artificial CpG-containing single-stranded oligodeoxynucleotides (ODNs), each of which is consisted of single-stranded oligodeoxynucleotide DNA molecule containing one or more CpG(s), wherein said ODNs can stimulate human peripheral blood mononuclear cell (PBMC) to produce antiviral substances. These ODNs can protect the cells against the attack from virus, wherein said virus is preferably selected from the group consisted of influenza virus and single-stranded positive strand RNA virus such as SARS virus, hepatitis C virus, dengue virus and Japanese encephalitis virus. Moreover, the antiviral use of artificial CpG ODNs and its use for treating and preventing viral infection are also provided.
Owner:CHANGCHUN HUAPU BIOTECHNOLOGY CO LTD
Methods for real-time multiplex isothermal detection and identification of bacterial, viral, and protozoan nucleic acids
ActiveUS10072309B1Flexible processFacilitate strand displacementMicrobiological testing/measurementAgainst vector-borne diseasesProtozoaFluorescence
Herein disclosed are rapid real-time isothermal multiplex methods of detecting, identifying and quantifying bacterial, viral, and protozoan nucleic acids in a sample. These include contacting the sample with two or more sets of pathogen-specific reverse transcription loop-mediated isothermal amplification primers and novel oligofluorophores specific for the target bacterial, viral, and parasitic nucleic acids of interest such as human immunodeficiency virus, Ebola virus, Marburg virus, Yellow fever virus, hepatitis-B virus, Lassa fever virus, Plasmodium, hepatitis-C virus, hepatitis-E virus, dengue virus, Chikungunya virus, Japanese Encephalitis virus, Middle Eastern Respiratory Syndrome Corona virus, Mycobacterium, West Nile virus, Cytomegalovirus, Parvovirus, Leishmania, Trypanosoma, and Zika virus nucleic acids, under conditions sufficient to produce detectable real-time amplification signals in about 10 to 40 minutes. The amplification signals are produced by pathogen-specific fluorogenic labels included in one or more of the primers. Also, novel reaction and sample lysis buffers, primers, and kits for rapid multiplex detection, quantification, and identification of bacterial, viral, and protozoan nucleic acids by real-time isothermal amplification are herein disclosed.
Owner:NYAN DOUGBEH CHRIS
Host targeted inhibitors of dengue virus and other viruses
ActiveUS9879003B2Delay or minimize one or more symptoms associatedReduces and avoids symptom and causeOrganic chemistryHerpes simplex diseaseJunin virus
Novel antiviral compounds of Formulae (I)-(III) are provided: (I) (II) (III) The inventive compounds, pharmaceutical compositions thereof, and kits including the inventive compounds are useful for the prevention and treatment of infectious diseases caused by viruses, for example, by Flaviviridae virus (e.g., Dengue virus (DENY)), Kunjin virus, Japanese encephalitis virus, vesicular stomatitis virus (VSV), herpes simplex virus 1 (HSV-1), human cytomegalovirus (HCMV), poliovirus, Junin virus, Ebola virus, Marburg virus (MARV), Lassa fever virus (LASV), Venezuelan equine encephalitis virus (VEEV), or Rift Valley Fever virus (RVFV).
Owner:DANA FARBER CANCER INST INC +1
Nucleic acid vaccines for prevention of flavivirus infection
InactiveUS7417136B1Easy to administerEasy to prepareOrganic active ingredientsVirusesActive agentEncephalitis Viruses
The invention encompasses nucleic acid molecules containing transcription units which encode the flavivirus M and E protein antigens. The flaviviruses include Japanese encephalitis virus, dengue, yellow fever virus and St. Louis encephalitis virus. The nucleic acids function to provide the M and E protein antigens when the nucleic acid resides in an appropriate host cell, especially when the host cell is the cell of a subject. The invention also encompasses a vaccine whose active agent is the nucleic acid. The invention further encompasses the cultured host cells when they contain within them nucleic acid molecules containing the transcription units. The invention in addition encompasses a method of immunizing a subject against flavivirus infection by administering to the subject an effective amount of a vaccine containing a nucleic acid molecule containing the transcription unit of the invention.
Owner:HEALTH & HUMAN SERVICES DEPT OF UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC THE
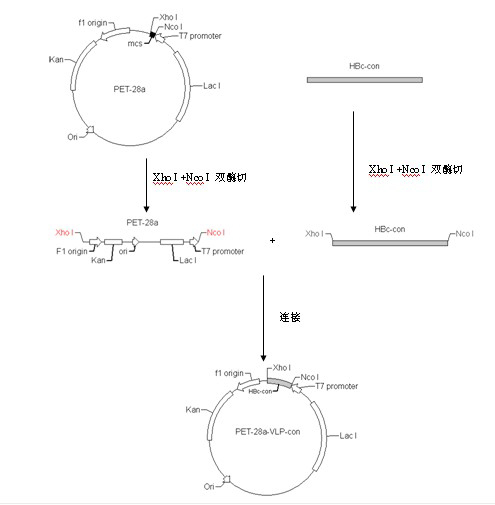
Japanese encephalitis particle vaccine and preparation method and application thereof
InactiveCN102127554AImprove the level ofStrong immune memoryViral antigen ingredientsVirus peptidesHepatitis B virus core AntigenEscherichia coli
The invention belongs to the field of biotechnology, and relates to a vaccine embedded with virus-like particles expressing multi-epitope antigen for Japanese encephalitis, and a preparation method and application thereof. The antigen of the vaccine is virus-like particles formed through spontaneous assembly of hepatitis B virus core antigen embedded with neutralizing antigen epitope expressing Japanese encephalitis virus and cytotoxic lymphocyte (CTL) antigen epitope, and is prepared through soluble expression of escherichia coli and purification. The Japanese encephalitis virus-like particle antigen is properly diluted with physiological saline, or is compatible with immunologic adjuvants to be prepared into the Japanese encephalitis particle vaccine. Animal experiments show that: the vaccine is safe and high-efficiency; mice inoculate with the vaccine generate high-level neutralizing antigen for the Japanese encephalitis virus to protect the mice against the attack of strong Japanese encephalitis virus by 100 percent.
Owner:NANJING AGRICULTURAL UNIVERSITY
ELISA (Enzyme-Linked Immuno Sorbent Assay) kit for detecting Japanese encephalitis virus antigens in swine, human and mosquitoes and application
InactiveCN102464716AEfficient detectionShort period of bloodMicroorganism based processesImmunoglobulins against virusesAnimal virusProtein.monoclonal
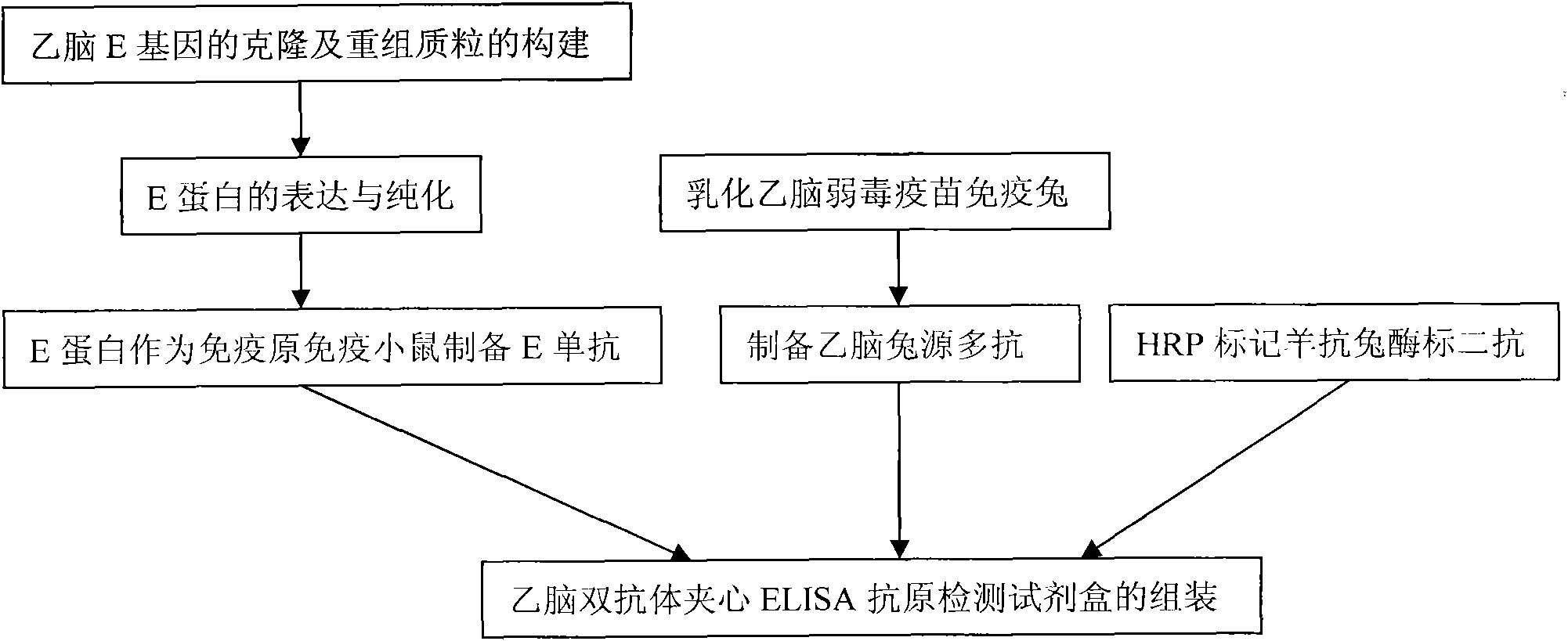
The invention belongs to the technical field of animal virology and immunology, in particular to a double-antibody sandwich ELISA (Enzyme-Linked Immuno Sorbent Assay) kit for detecting Japanese encephalitis virus antigens in swine, human and mosquitoes. Core reagents of the kit comprise a Japanese encephalitis virus E protein monoclonal antibody serving as a primary antibody and a rabbit polyclonal antibody serving as a secondary antibody. The invention discloses a preparation and purification method of the Japanese encephalitis virus E protein monoclonal antibody and the rabbit polyclonal antibody. The invention also discloses a detection method of the double-antibody sandwich ELISA. A hybridoma cell strain secreting the monoclonal antibody is preserved in China Center for Type Culture Collection with CCTCC NO. C2010114.
Owner:HUAZHONG AGRI UNIV
Enhanced immunogen for inactivated vaccine for infection with Japanese encephalitis viruses and process for producing the same
InactiveUS6841374B1Low production costSsRNA viruses positive-senseViral antigen ingredientsDiagnostic agentTiter
The present invention provides a novel inactivated virus particle and a reinforced immunogen which have a reinforced titer about twice to about 10 times that of a conventional vaccine, as well as a method for producing the same. The inactivated virus particle is produced by inactivating and then purifying a culture by physical means. The inactivated virus particle of the present invention is useful in a diagnostic agent for infectious disease caused by a group of Japanese encephalitis virus.
Owner:THE RES FOUND FOR MICROBIAL DISEASES OFOSAKA UNIV
Novel antiviral medicines and application thereof
PendingCN108721281ABroad-spectrum and excellent antiviral activityLow toxicityAntiviralsNitrile/isonitrile active ingredientsLeflunomideRNA virus
The invention discloses application of leflunomide, teriflunomide, brequinar and derivatives thereof to treatment of virus infection, especially RNA virus infection. RNA viruses include but are not limited to influenza viruses, respiratory syncytial viruses, hand, foot and mouth viruses (EV71), dengue viruses (type-2 dengue viruses), Zika viruses and Japanese encephalitis viruses. The medicines have broad-spectrum and excellent antiviral activity and have relatively low toxicity to normal cells.
Owner:EAST CHINA UNIV OF SCI & TECH
Antiviral traditional Chinese medical composition
ActiveCN101618193ALow toxicityDefinite curative effectAntiviralsAluminium/calcium/magnesium active ingredientsSide effectForsythia
The invention relates to the field of traditional Chinese medicines and provides an antiviral traditional Chinese medical composition comprising the following raw materials based on parts by weight: 350-370 parts of isatis roots, 150-170 parts of gypsum, 80-100 parts of radix rehmanniae, 70-90 parts of patchouli, 120-140 parts of weeping forsythia, 160-180 parts of reed roots, 60-80 parts of radix curcumae, 60-80 parts of grass-leaved sweetflag and 60-80 parts of rhizoma anemarrhenae. Clinical experiments prove that the traditional Chinese medical composition has a broad antiviral spectrum, can inhabit influenza virus, herpes simplex virus, Japanese encephalitis virus, respiratory syncytial virus, adenovirus, coxsackie virus, SARS virus, parainfluenza virus and newcastle disease virus and has high effective rate and safety without toxic and side effects, thereby having wide clinical application prospects.
Owner:吉林福辉药业股份有限公司
Methods of treatment and diagnosis using modulators of virus-induced cellular gene sequences
InactiveUS20070087982A1Reduce expressionReducing and preventing expression of mRNABiocideGenetic material ingredientsDiseaseImmunodeficiency virus
Applicants have used microarrays, gene expression profiling, and gene silencing methods to identify and provide a plurality of ‘validated’ virus-induced cellular gene sequences (e.g., HMG20B, HRH1, NP and c-YES (src family kinases)) and pathways useful as therapeutic targets for modulation of viral-mediated cellular effects. Particular embodiments provide therapeutic compositions, and methods for modulation of viral infection, replication, maturation, progression, or other virally-related conditions or diseases, comprising inhibition of virally-induced gene sequences and gene products. Additional embodiments provide screening assays for compounds useful to modulate viral infection, replication, maturation or progression, or viral-related conditions or diseases. Further embodiments provide diagnostic and / or prognostic assays for viral infection, replication, maturation or progression. Preferably, the viruses all selected from the group consisting of retroviruses (e.g., human immunodeficiency virus (HIV), and viruses of the family Flaviviridae that includes the flaviviruses (e.g., West Nile virus (WNV), Japanese encephalitis virus (JEV), yellow fever virus (YFV) and Dengue fever virus (DEN)), and hepatitis C virus (HCV).
Owner:OREGON HEALTH & SCI UNIV
Vaccine composition, preparation method and application thereof
ActiveCN104043117AReduce the impactLess side effectsViral antigen ingredientsAntiviralsDiseaseSide effect
The invention provides a vaccine composition, a preparation method and an application thereof. The vaccine composition includes: an immune dose of porcine circovirus 2-type antigen, an immune dose of porcine Japanese encephalitis virus antigen, an immune dose of porcine parvovirus antigen and an adjuvant. The vaccine composition can effectively prevent and cure postweaning piglet multisystemic syndrome and sow reproduction dysfunctional diseases which are caused by 2-type porcine circovirus, porcine Japanese encephalitis virus and porcine parvovirus. An immune effect of the vaccine composition is better than that of a single vaccine. The vaccine composition is little in side effects, is high in valence of a serum antibody, has a long immune period, can save time and labor intensity, has a small stress response to a pig, can simplify immunity processes and can reduce production cost and prevention cost.
Owner:PU LIKE BIO ENG
Flavivirus inhibitors and methods for their use
Methods of treating, preventing, and / or ameliorating a Flavivirus infection in a subject are disclosed. The methods comprise administering to the subject a therapeutically effective amount of a Flavivirus inhibitor, e.g., a Flavivirus serine protease inhibitor. These methods are useful in treating, preventing, and / or ameliorating Flavivurs infections such as, for example, West Nile Virus, Dengue Virus, and Japanese Encephalitis Virus.
Owner:GEORGETOWN UNIV
Japanese encephalitis vaccine prepared by human embryonic lung fibroblasts and preparation method thereof
ActiveCN101524536AFully identifiedFully standardizedViral antigen ingredientsAntiviralsImmune effectsJapanese encephalitis vaccine
The invention discloses a Japanese encephalitis vaccine prepared by human embryonic lung fibroblasts and a preparation method thereof, comprising culture and expansion of the human embryonic lung fibroblasts. The method comprises the following steps: Japanese encephalitis virus strain P3, strain SA14-14-2 or strain Nakayama is naturalized and inoculated to fit the human embryonic lung fibroblasts, and the seeds of Japanese encephalitis viruses are prepared on the human embryonic lung fibroblasts; wherein, an inactivated Japanese encephalitis vaccine also comprises the steps of harvesting viruses, inactivating viruses, concentrating, purifying and the like; an attenuated live vaccine also comprises the steps of harvesting viruses, concentrating, purifying and the like. Due to being prepared by healthy human embryonic lung fibroblasts, the two kinds of Japanese encephalitis vaccines do not contain any adventitious pollution agent and tumorigenicity, has high purity after being purified and has the advantages of good immune effect and high security. The preparation method of the invention is suitable for large-scale industrial production and can meet the preparation processes of Japanese encephalitis vaccines required by domestic and abroad markets.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
Hetero type pentamer recombinant vaccine
A heteropentamer composed of a fusion monomer (32) comprising a fusion protein of an antigenic amino acid sequence and an amino acid sequence of a monomer of a mucous membrane-binding protein and a nonfusion monomer (20) of an amino acid sequence of a monomer of a mucous membrane-binding protein. A homopentamer composed of a fusion monomer including a fusion protein of an antigen derived from an envelope protein of Japanese encephalitis virus and an amino acid sequence of a monomer of a mucous membrane-binding protein. These heteropentamer and the homopentamer can be used as a vaccine.
Owner:ADVANCED MEDICAL BIOLOGICAL SCI INST +1
EIII-indirect ELISA (Enzyme-Linked Immuno Sorbent Assay) antibody detection kit for detecting swine Japanese encephalitis virus and application thereof
ActiveCN103616509AImproving immunogenicityEvade attackBiological material analysisElisa kitJapanese encephalitis virus Antibody
The invention discloses an EIII-indirect ELISA (Enzyme-Linked Immuno Sorbent Assay) antibody detection kit for detecting a swine Japanese encephalitis virus and an application thereof. The kit disclosed by the invention consists of an elisa plate which takes a purified JEV-EIII protein as an envelope antigen, a cleaning liquid, a serum diluent, rabbit anti-pig elisa secondary antibody, a primer developing liquid, a stop buffer, a JEV positive serum and a JEV negative serum. The envelope antigen used by the invention is easy to be obtained stably and massively, the purifying method is simple and easy to realize, and concentration of recombinant proteins is easy to test and control, thereby facilitating industrialized production on a large scale. The kit disclosed by the invention is used for detecting the swine Japanese encephalitis virus antibody, and the detection coincidence rate with the ELISA kit in the prior art is 90%. The kit disclosed by the invention is convenient to operate, high in sensitivity, good in specificity, low in using cost, good in repeatability and suitable for popularization and application, and provides a reliable means for clinical rapid detection of the JEV antibody.
Owner:广州易安生物技术有限公司
Recombinant flaviviral constructs and uses thereof
InactiveUS20130243809A1Successfully expressingHigh insertion capacitySsRNA viruses positive-senseViral antigen ingredientsStructural proteinViral replication
A recombinant viral construct for expressing an exogenous polypeptide in a cell and uses thereof are provided. The recombinant viral constructs are derived from Japanese encephalitis virus (JEV). The recombinant viral constructs encodes a fusion protein, which includes an exogenous (i.e., non-JEV) polypeptide and a JEV non-structural protein 1 (JEV NS1) or a segment thereof. Particularly, the exogenous polypeptide is inserted into the carboxyl-terminus of the JEV NS1, and the production of the recombinant fusion protein does not affect viral replication. Upon infection a cell with such recombinant viral constructs, JEV particles comprising limited multiplicative virions (LMV) may be produced. Each LMV comprises the as-described JEV replicon. The JEV particles are useful in eliciting an immune response to the exogenous polypeptide in a host and thereby confer the host with protective immunization against the exogenous polypeptide.
Owner:NAT DEFENSE MEDICAL CENT
Competitive enzyme linked immunosorbent assay (C-ELISA) for the detection of a flavivirus specific antibody
InactiveCN101449165ASsRNA viruses positive-senseBiological material analysisSpecific immunitySecondary Infections
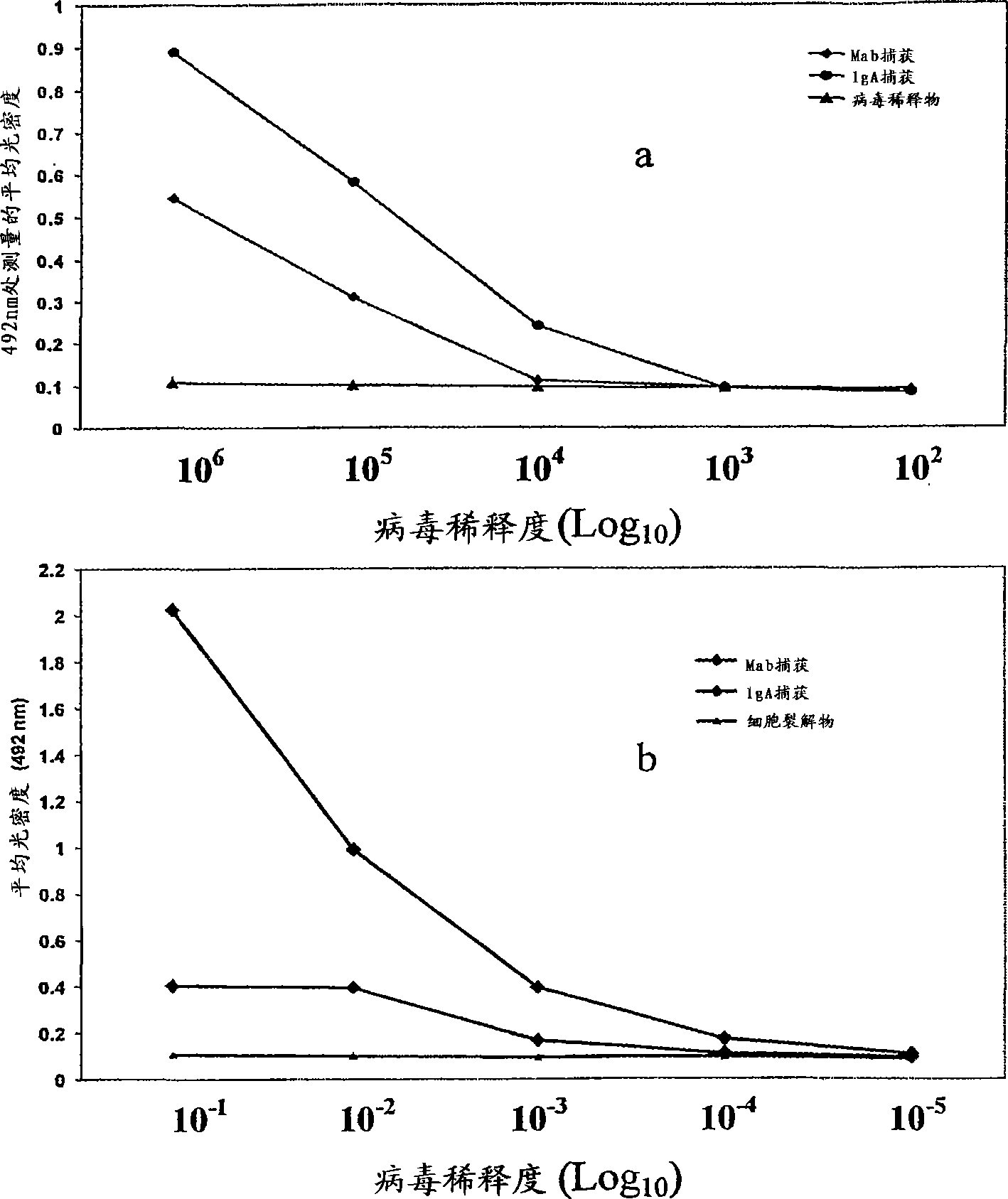
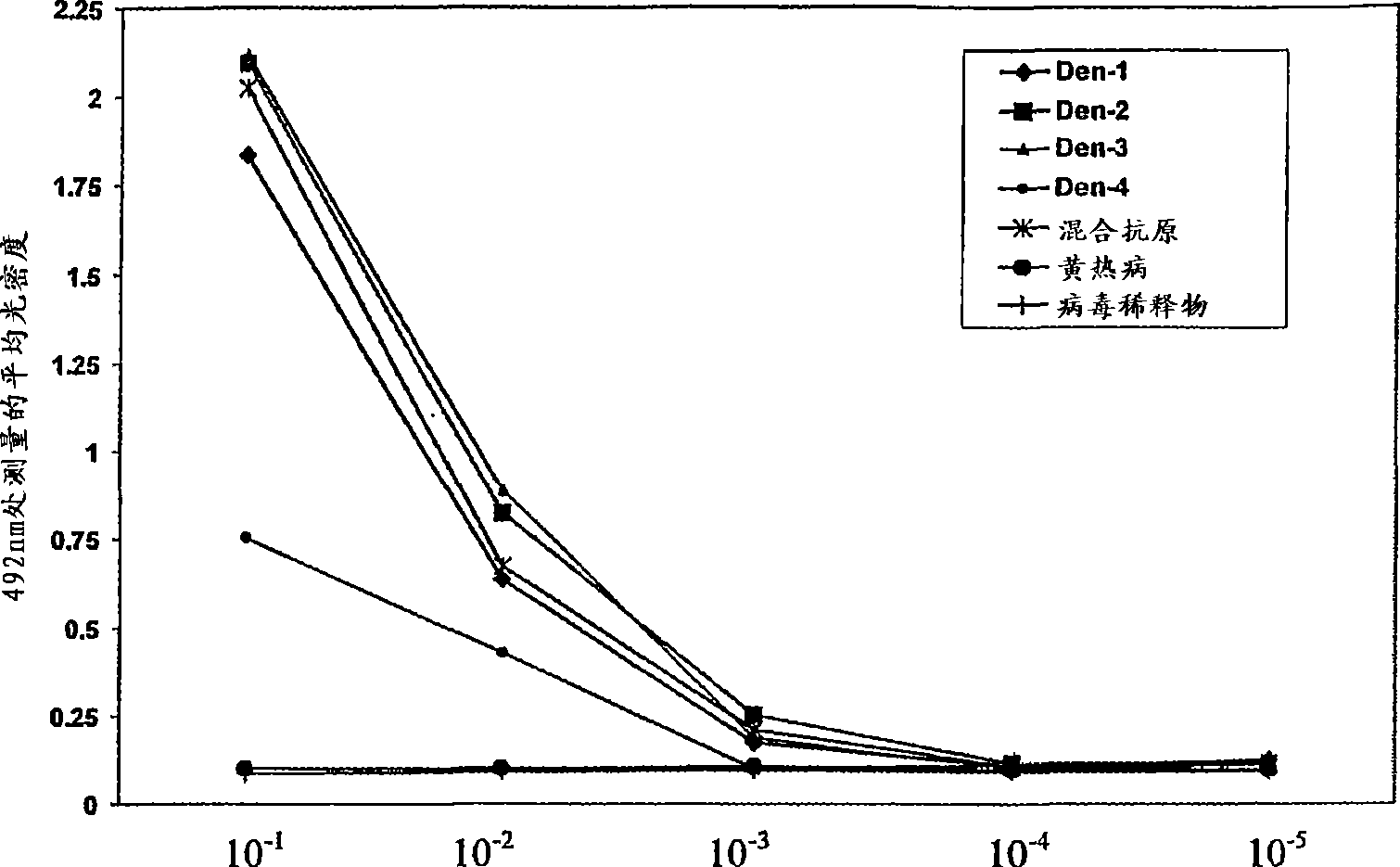
A competitive enzyme-linked immunosorbent assay (C-ELISA), using flavivirus member specific immunological agents was developed to detect antibody specific to members of the flaviviruses indicative of exposure to flavivirus. The test is based on a competition for epitope binding on the envelope protein of the flavivirus antigen captured using anti-flavivirus IgA in the presence of flavivirus positive serum. This test has comparable sensitivity specificity and speed to the virus neutralization assay (VNT). C-ELISA is a versatile technique, which could have various applications. Slight modifications of this protocol could lead to a C-ELISA-based detection method of secondary infection or one that could be used for serotype specific sero-epidemiological studies and / or vaccine evaluation. The protocol developed for C-ELISA was demonstrated using dengue lysate antigen and dengue specific monoclonal antibody. This can be used against other flaviviruses and the results for Japanese encephalitis illustrates this.
Owner:NATIONAL ENVIRONMENT AGENCY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com