Vaccine composition, preparation method and application thereof
A vaccine composition and immunogenicity technology, applied in the field of vaccine compositions, can solve the problems of increased stress response of pigs, increased risk of infection, complicated operation procedures, etc., to avoid adverse reactions and to last for a long immunization period. , the effect of simplifying the immunization program
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1, Porcine Circovirus, Porcine Japanese Encephalitis Virus and Porcine Parvovirus Triple Inactivated Vaccine Composition, Preparation and Inspection of Porcine Japanese Encephalitis Virus and Porcine Parvovirus Dual Vaccine
[0039] 1. The source of the virus strain
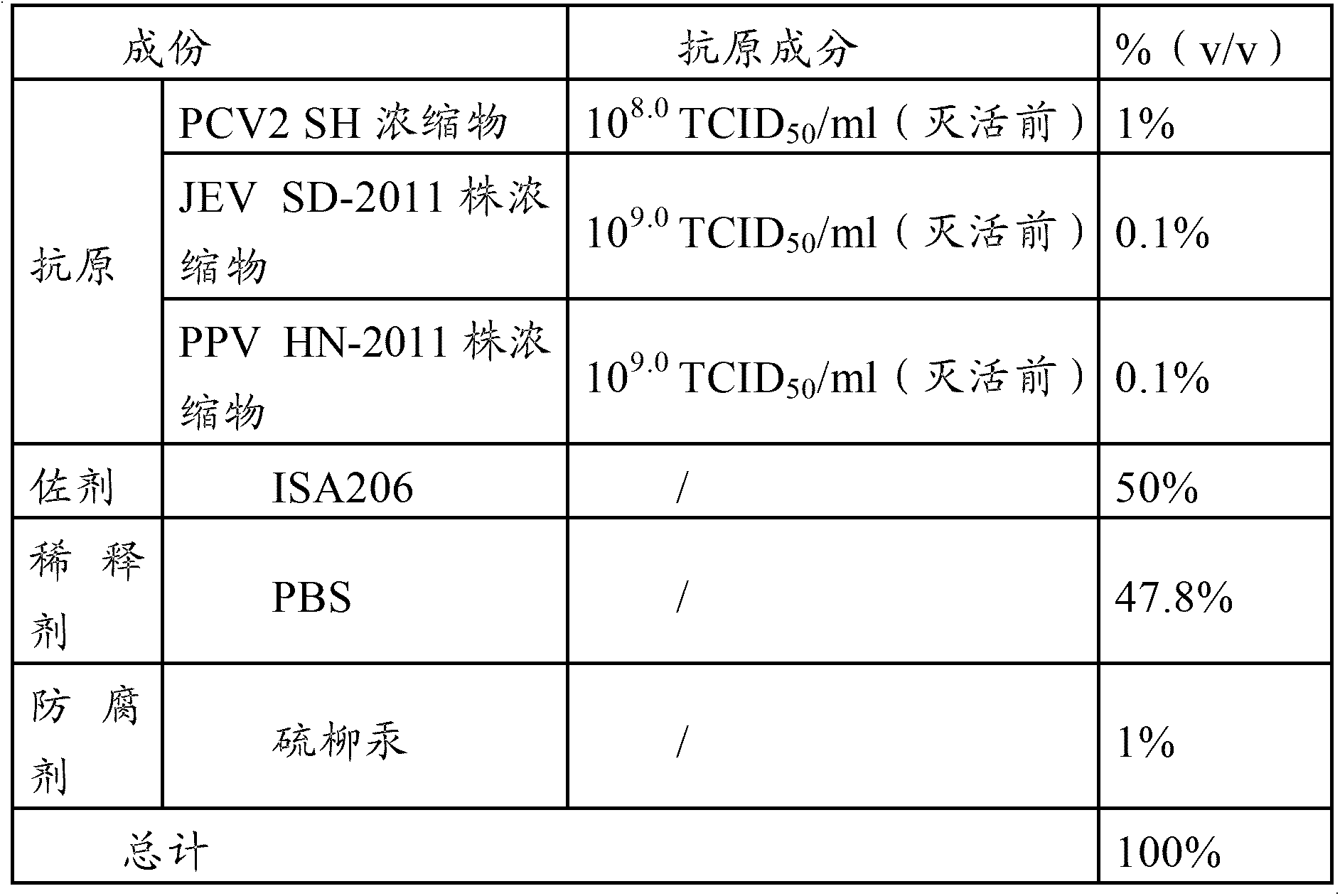
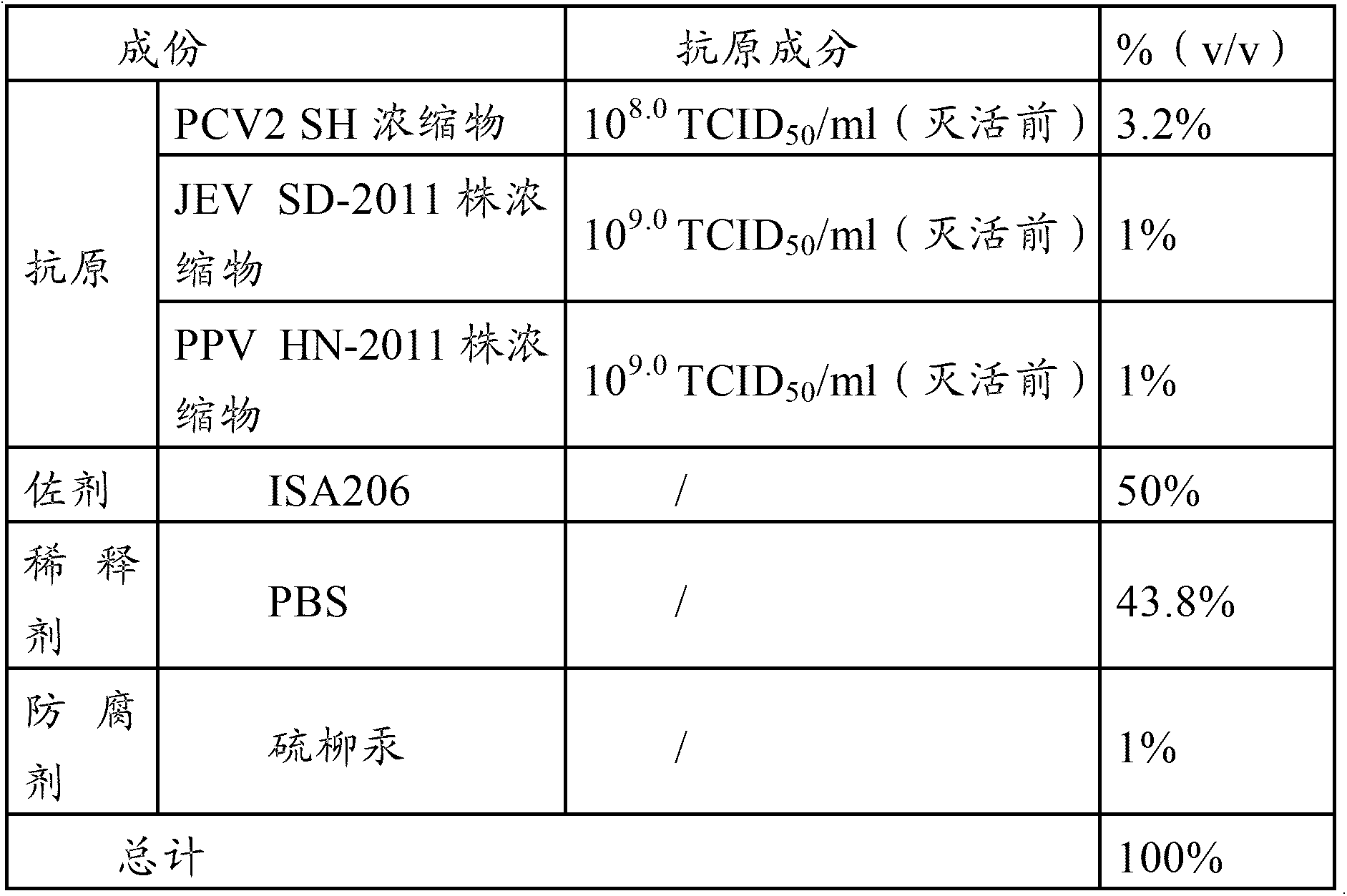
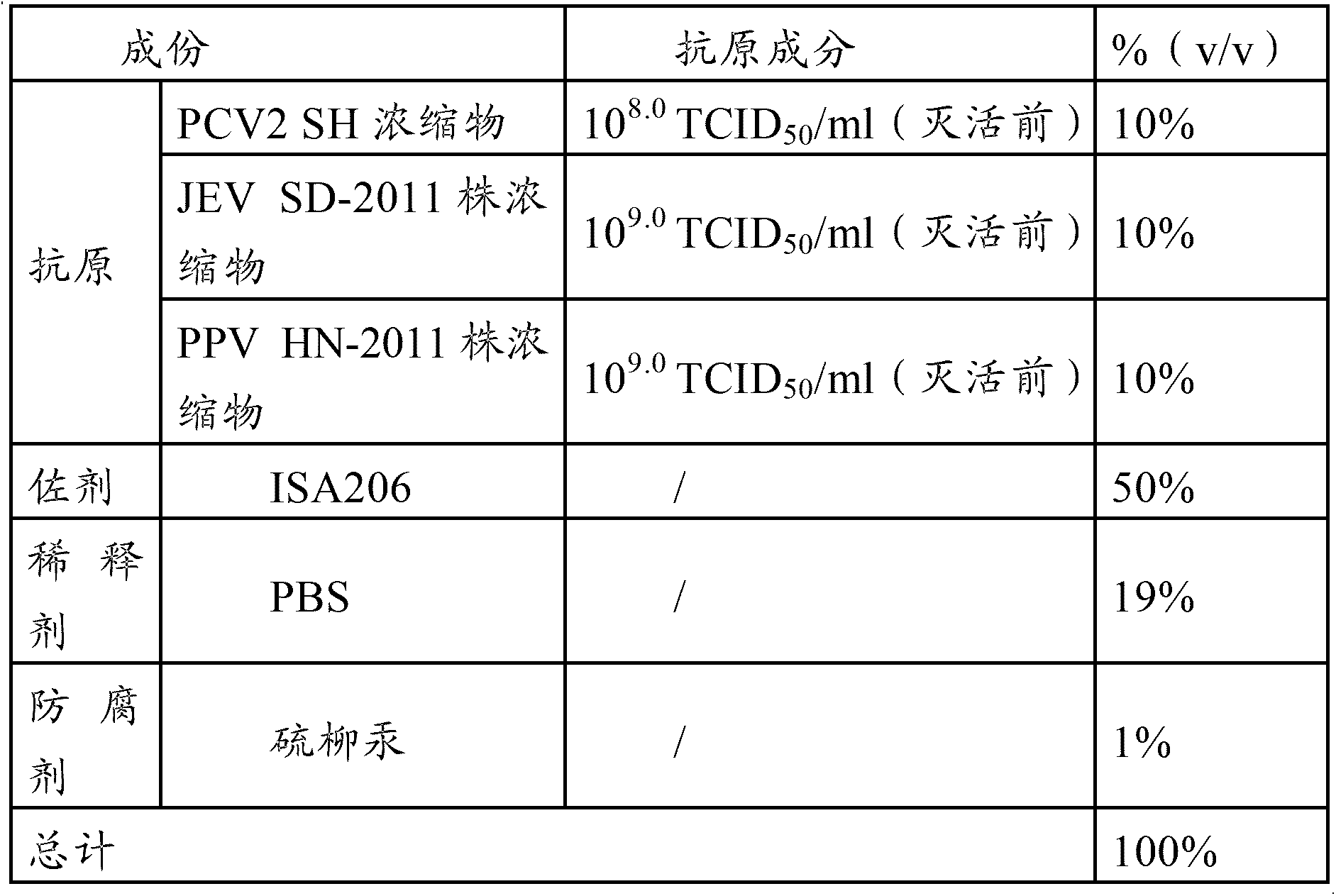
[0040] The PCV2 virus used in the PCV2-PPV-JEV triple vaccine in the embodiments of the present invention is the PCV2 SH strain, and the JEV strain used in the PCV2-PPV-JEV triple vaccine and the PPV-JEV double vaccine in the embodiments of the present invention is The SD-2011 strain, the PPV strain used in the PCV2-PPV-JEV triple vaccine and the PPV-JEV dual vaccine in the embodiment of the present invention is the HN-2011 strain.
[0041] 2. Preparation and inspection of vaccine semi-finished products
[0042] 2.1 Preparation of poisonous seeds for production
[0043] 2.1.1 Preparation of porcine circovirus type 2 SH strain:
[0044] Properly dilute the virus seed of PCV2 SH strain with virus ...
Embodiment 2
[0113] Example 2, this study is to evaluate the immune efficacy of PCV2-PPV-JEV combination triple vaccine with different antigen content and PPV-JEV dual vaccine and PCV2 single vaccine
[0114] 1. Test material
[0115] According to the method in embodiment 1, prepare PCV2-PPV-JEV triple vaccine (PCV2 antigen content is 10 6.0 TCID 50 / ml, PPV is 10 6.0 TCID 50 / ml, JEV is 10 6.0 TCID 50 / ml), recorded as vaccine L; PCV2-PPV-JEV triple vaccine (PCV2 antigen content is 10 6.5 TCID 50 / ml, PPV antigen content is 10 7.0 TCID 50 / ml, JEV antigen content is 10 7.0 TCID 50 / ml), recorded as vaccine M; PCV2-PPV-JEV triple vaccine (PCV2 antigen content is 10 7.0 TCID 50 / ml, PPV antigen content is 10 8.0 TCID 50 / ml, JEV antigen content is 10 8.0 TCID 50 / ml), recorded as vaccine H; low-dose PCV2-PPV-JEV triple vaccine (PCV2 antigen content is 5×10 5.5 TCID 50 / ml, PPV antigen content is 10 7.0 TCID 50 / ml, JEV antigen content is 10 7.0 TCID 50 / ml), recorded a...
Embodiment 3
[0144] Example 3. This study is to evaluate the reproductive performance and growth performance of piglets after PCV2-PPV-JEV triple combination vaccine and PPV-JEV dual vaccine combined with PCV2 single vaccine immunized sows
[0145] 1. Test material
[0146] According to the method in embodiment 1, prepare PCV2-PPV-JEV triple vaccine (PCV2 antigen content is 10 6.5 TCID 50 / ml, PPV antigen content is 10 7.0 TCID 50 / ml, JEV antigen content is 10 7.0 TCID 50 / ml), recorded as vaccine M; PCV2 inactivated vaccine (PCV2 antigen content is 10 6.5 TCID 50 / ml) is recorded as vaccine 2; PPV-JEV dual vaccine (PPV antigen content is 10 7.0 TCID 50 / ml, JEV antigen content is 10 7.0 TCID 50 / ml) was recorded as vaccine 3.
[0147] 2. Design of animal experiments
[0148] 2.1 Design of challenge experiment: 30 gilts were selected and divided into 3 groups with 10 gilts in each group. Before immunization, blood was collected from sows in each group for detection of viremia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com