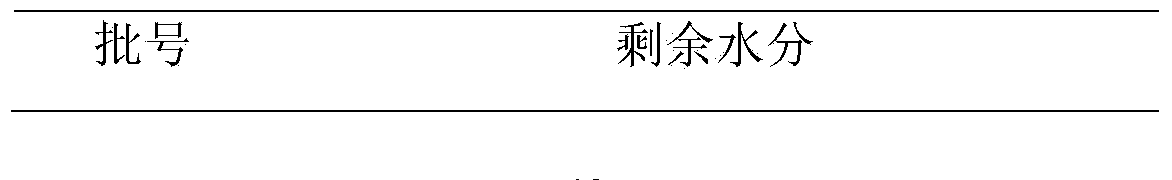
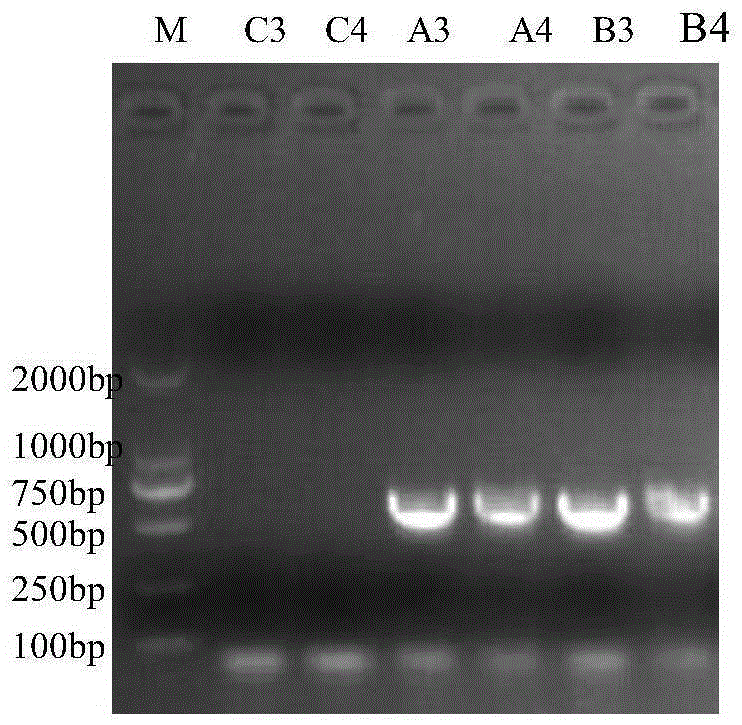
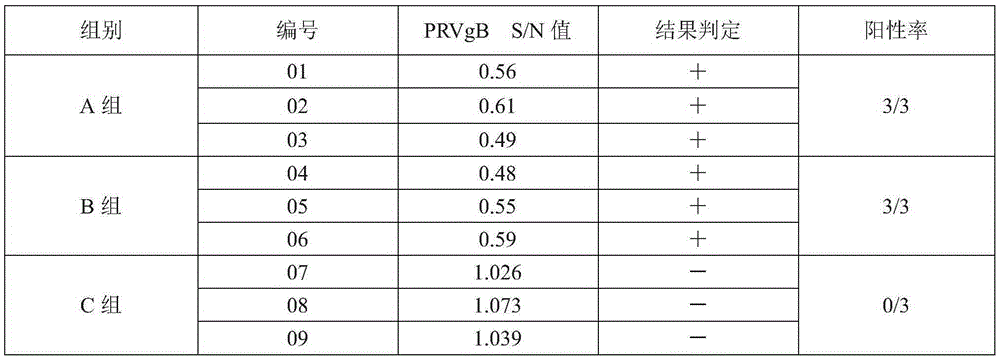
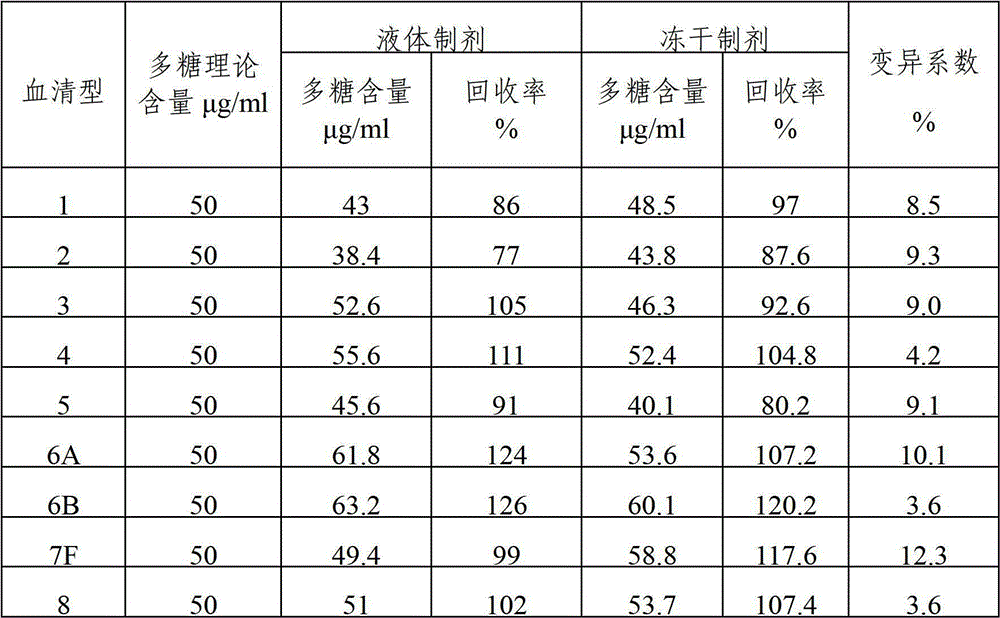
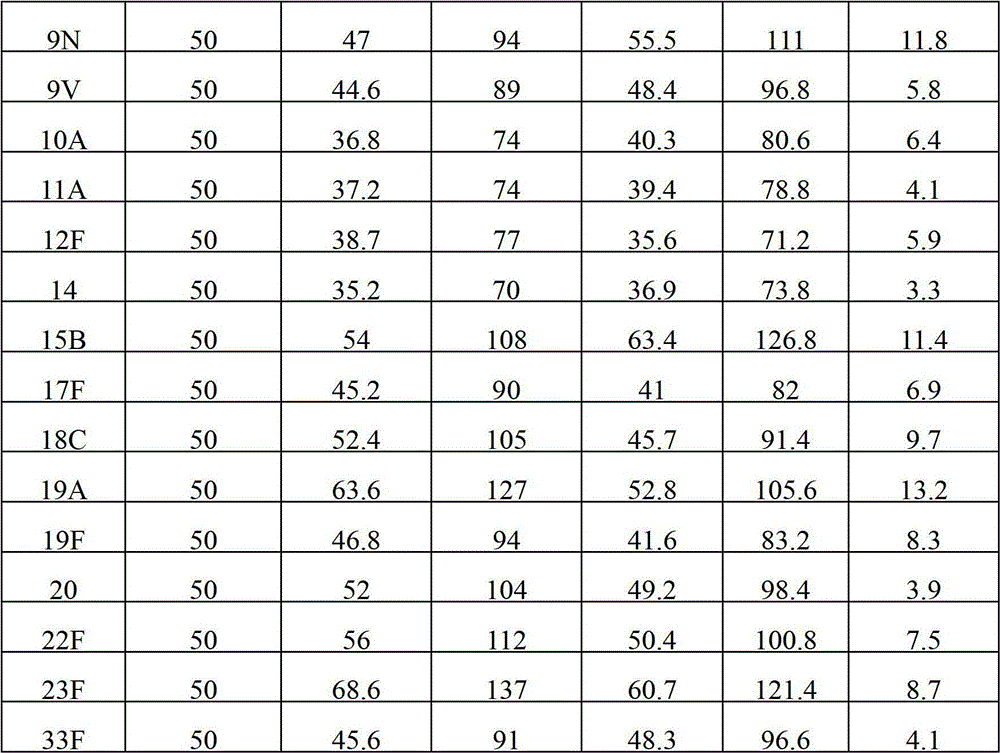
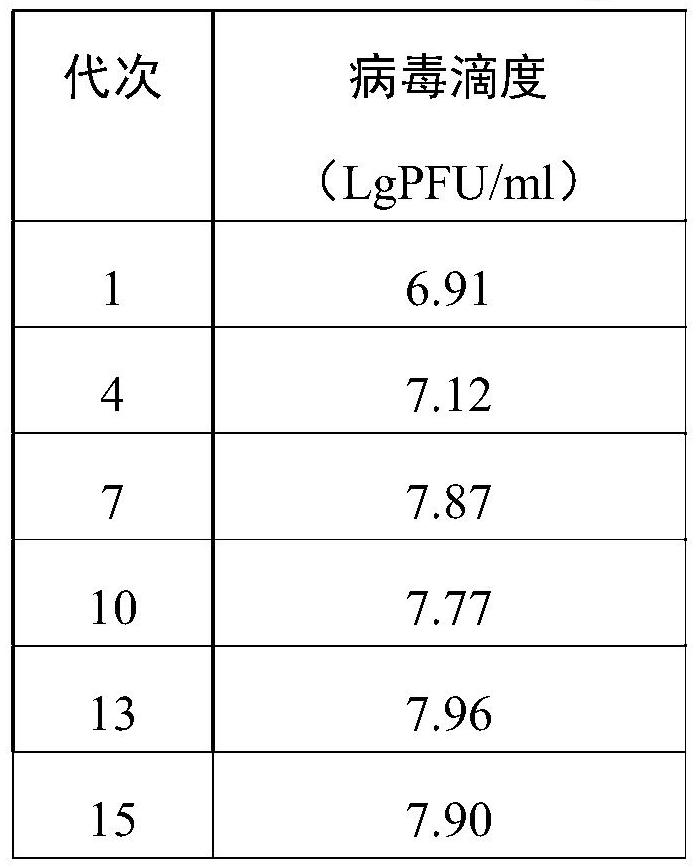
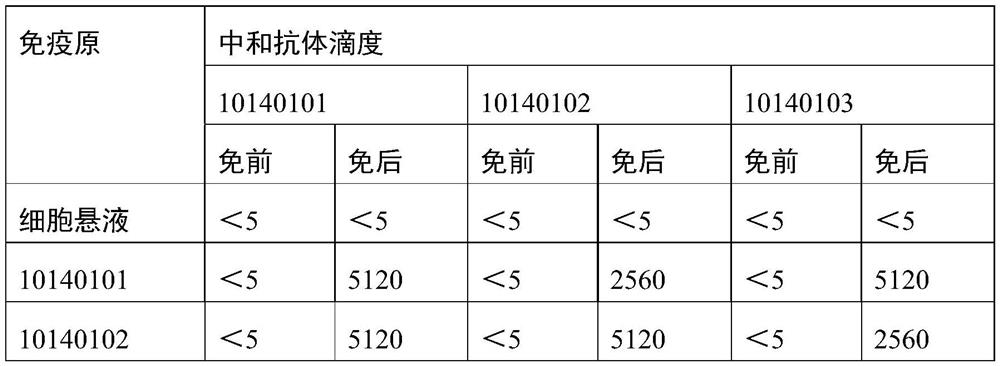
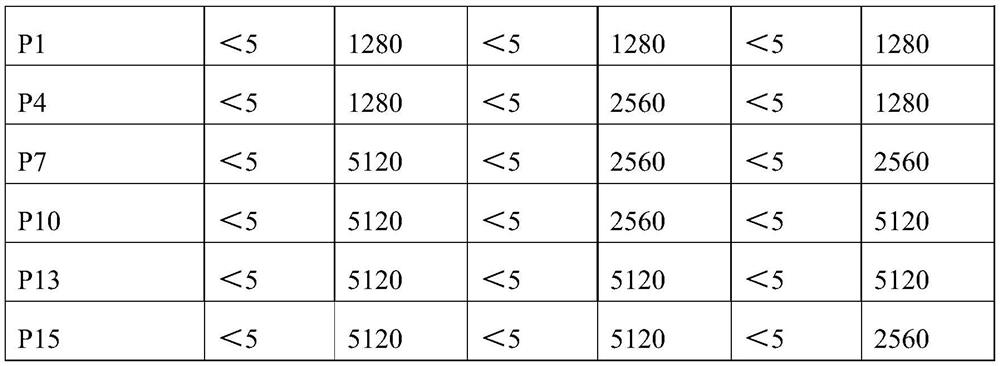
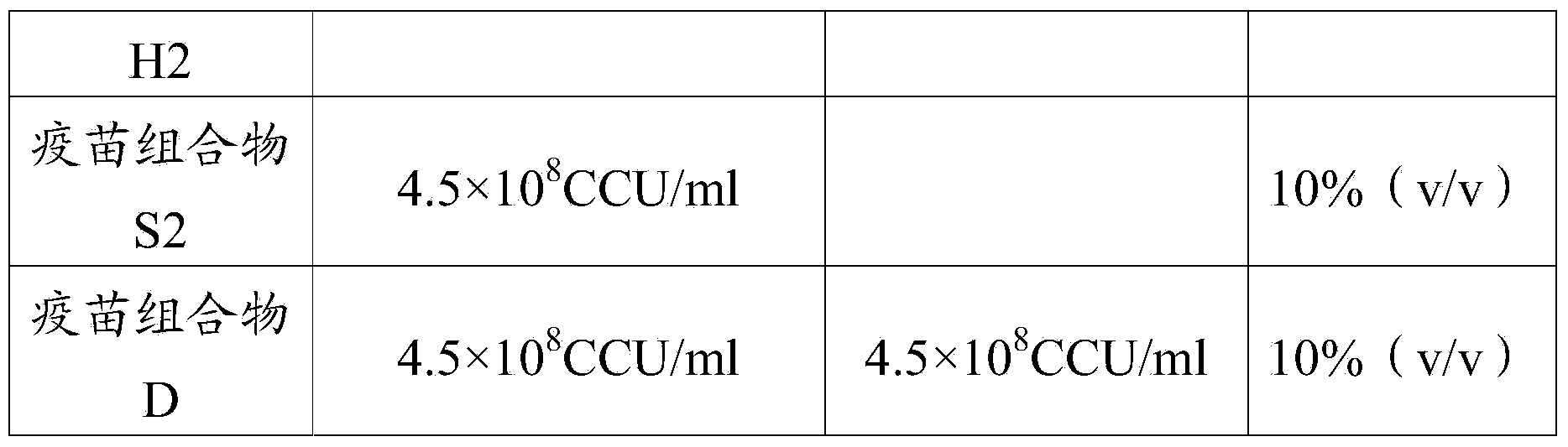Patents
Literature
40results about How to "Simplified immunization program" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
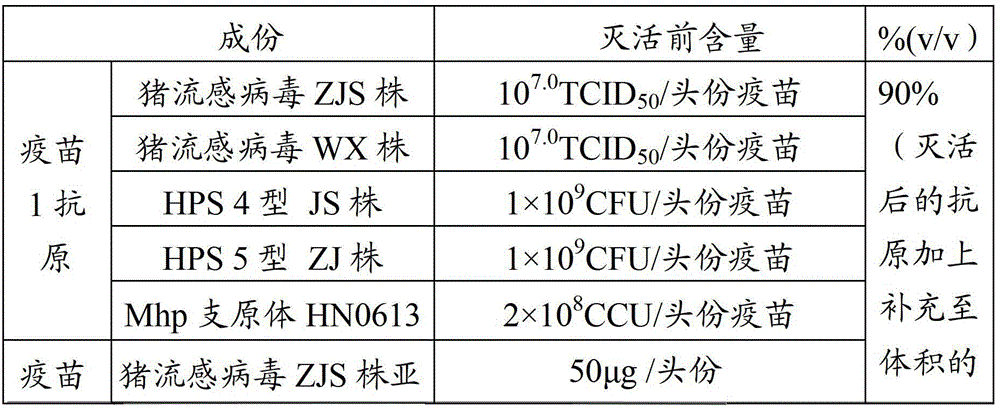
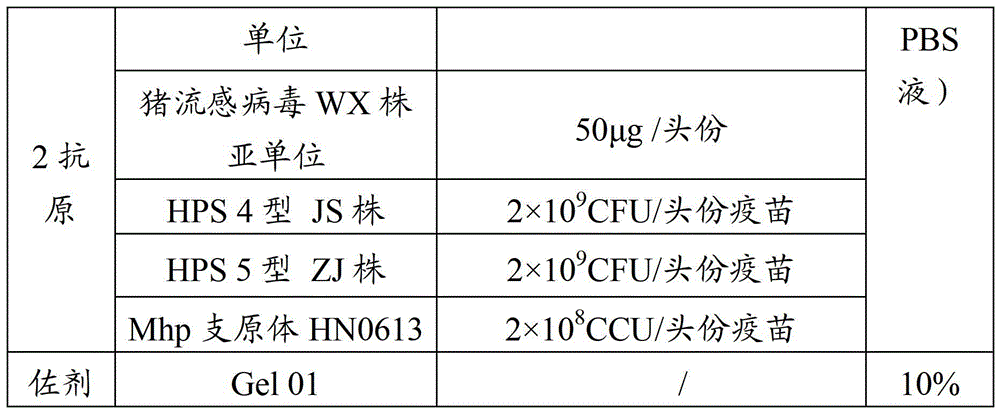
Vaccine composition for preventing and treating porcine circovirus type 2, haemophilus parasuis and mycoplasma hyopneumoniae infection and preparation method thereof
ActiveCN103083655ASimplified immunization programReduce manufacturing costAntibacterial agentsBacterial antigen ingredientsDiseaseCircovirus
The invention provides a vaccine composition for preventing and treating porcine circovirus type 2, haemophilus parasuis and mycoplasma hyopneumoniae infection. The vaccine composition comprises an inactivated porcine circovirus type 2 antigen, inactivated haemophilus parasuis, inactivated mycoplasma hyopneumoniae and a vaccine adjuvant. The vaccine composition disclosed by the invention can realize the aim of preventing three diseases including a porcine circovirus disease, mycoplasma pneumonia, a haemophilus parasuis disease by one injection of the vaccine; the content of antigen is 1 / 2 of the content of a common single-vaccine antigen when the vaccine composition disclosed by the invention is prepared by mixing the three antigens; and compared with the existing condition that three injections of single vaccine are injected to prevent three infectious diseases, the technical scheme disclosed by the invention is economical and practical, reduces the production cost, simplifies an immune procedure and reduces the epidemic prevention cost.
Owner:PU LIKE BIO ENG
Multivalent pneumococcal capsular polysaccharide composition as well as preparation method and application thereof
ActiveCN103656632AStable physical and chemical propertiesPrevent diseaseAntibacterial agentsBacterial antigen ingredientsConjugate vaccineStreptococcus pneumoniae capsular polysaccharide
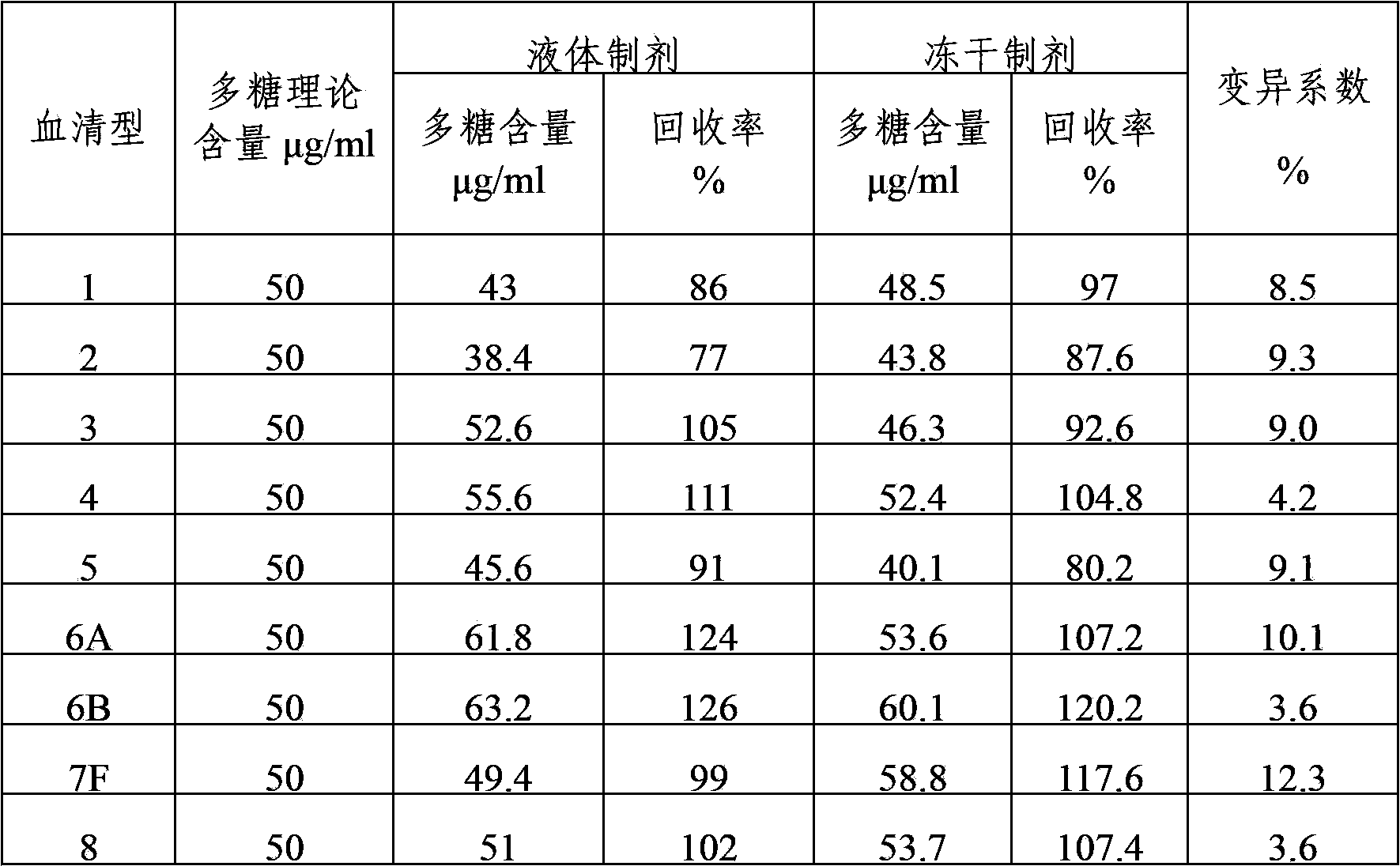
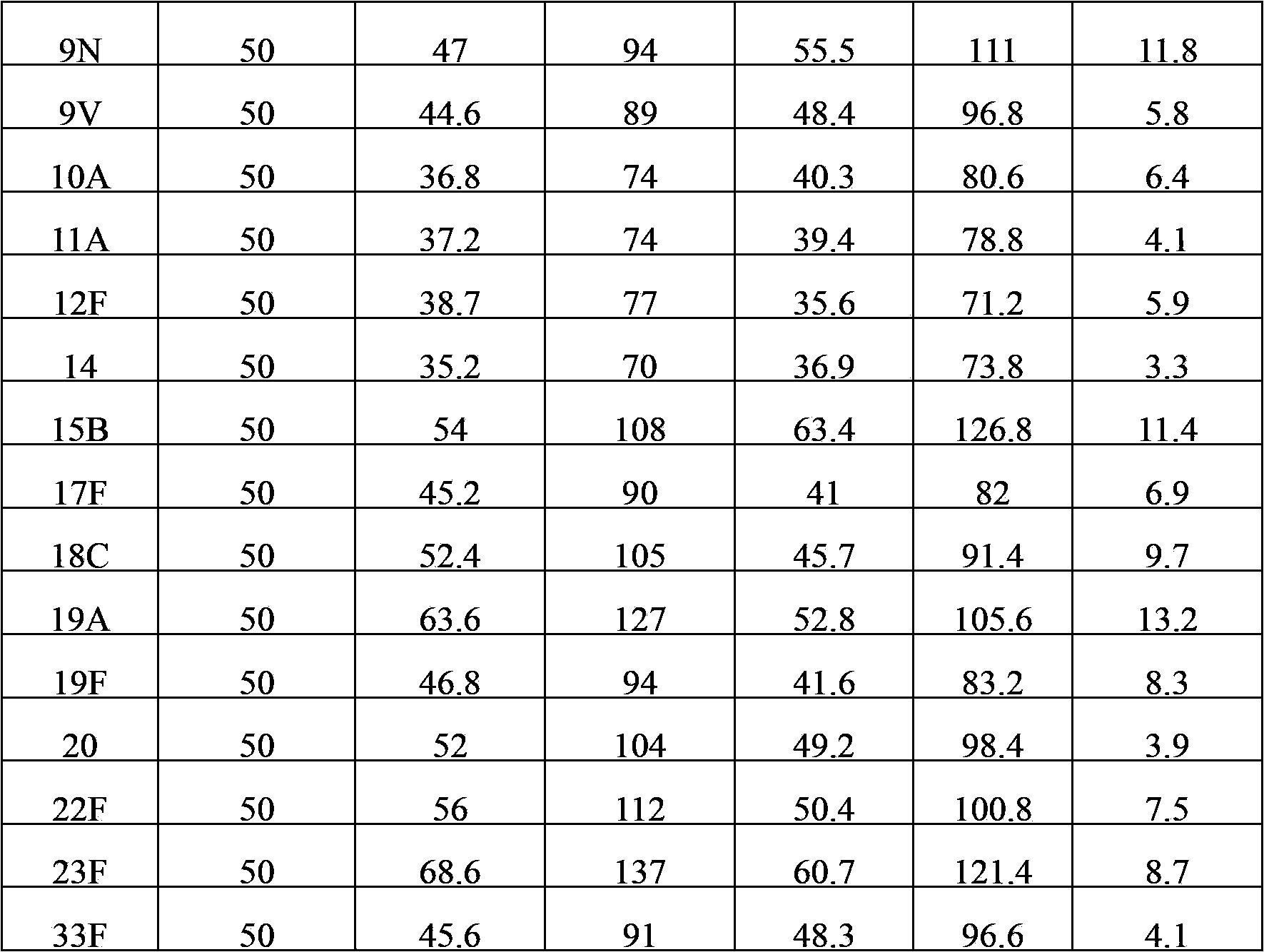
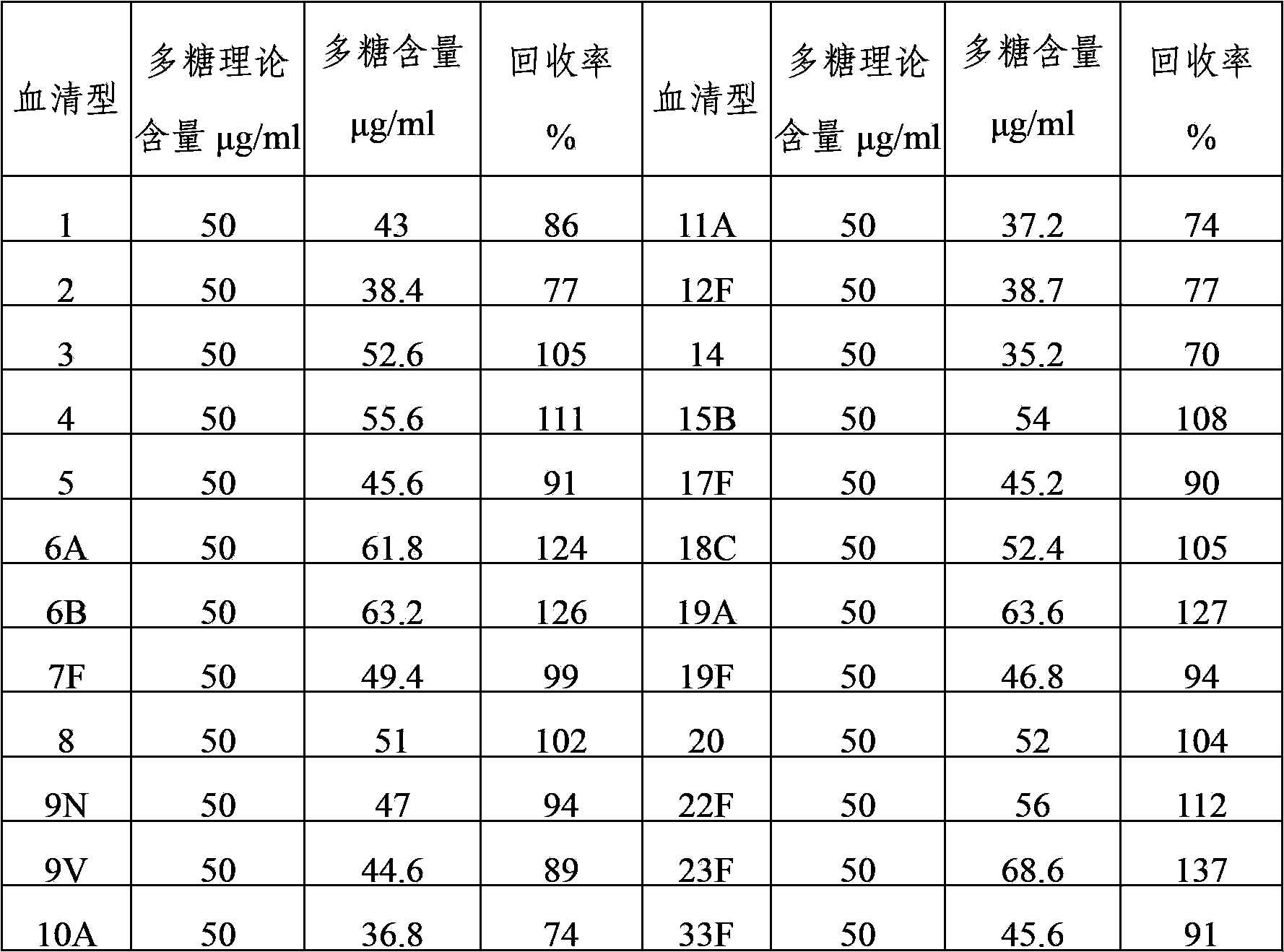
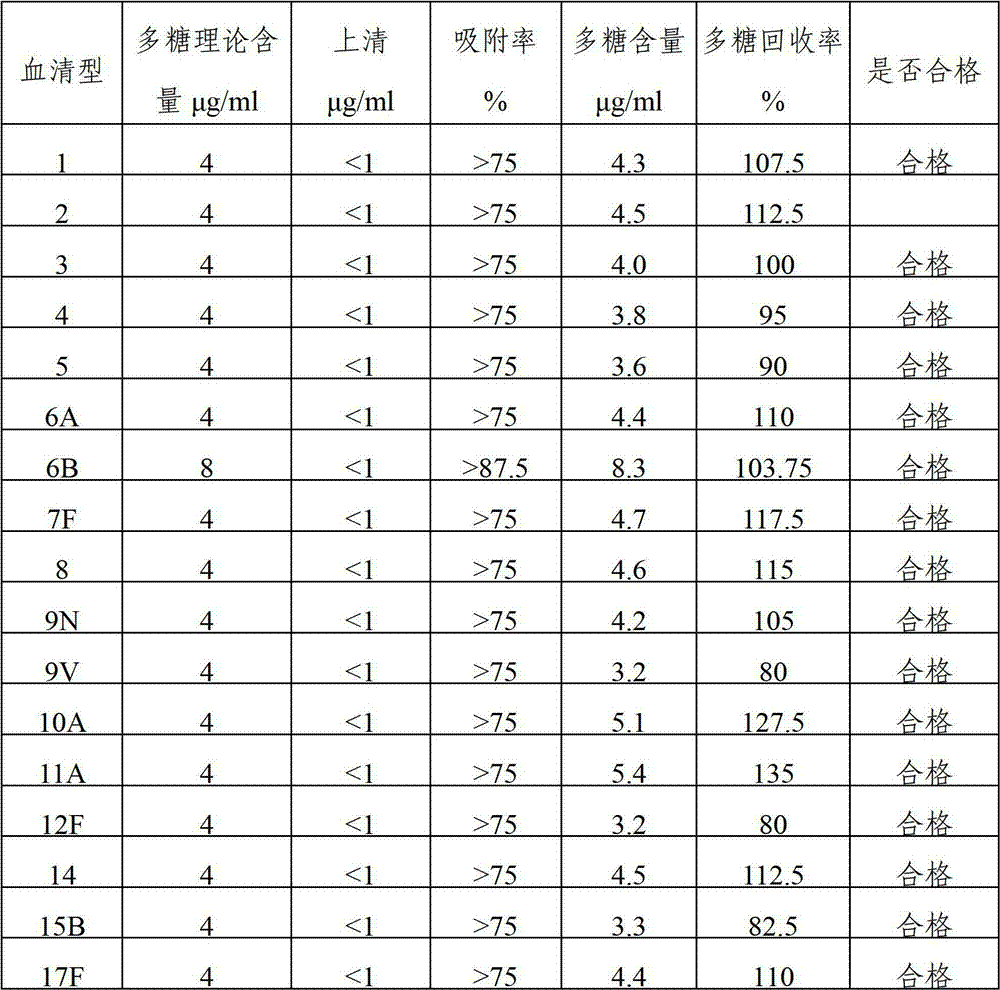
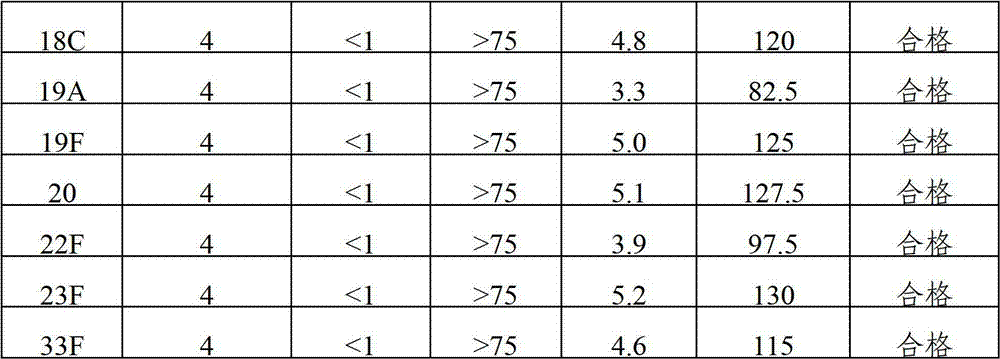
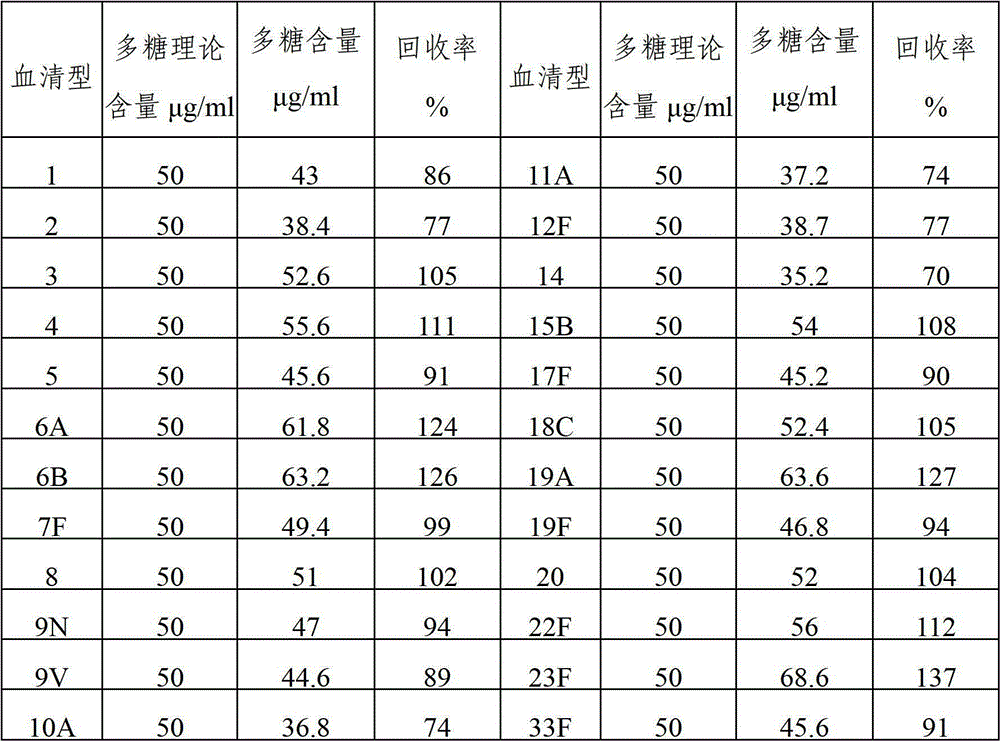
The invention provides a multivalent pneumococcal capsular polysaccharide composition as well as a preparation method and application thereof. The multivalent pneumococcal capsular polysaccharide composition contains a serotype 6A and at least one extra serotype selected from the group consisting of 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F. The multivalent pneumococcal capsular polysaccharide composition provided by the invention can be used for inducing an organism to generate humoral immunity, can generate a relatively good protecting effect for infectious diseases caused by the 24 common serotype pneumococcuses and is wide in immunity coverage rate and better in effect as comparison with various existing pneumococcal polysaccharide vaccines and conjugate vaccines sold on the market.
Owner:SINOVAC RES & DEV
Multivalent pneumococcus capsular polysaccharide-protein conjugated composition and preparation method thereof
InactiveCN104069488AImprove adsorption capacityImprove stabilityAntibacterial agentsBacterial antigen ingredientsDiseaseConjugate vaccine
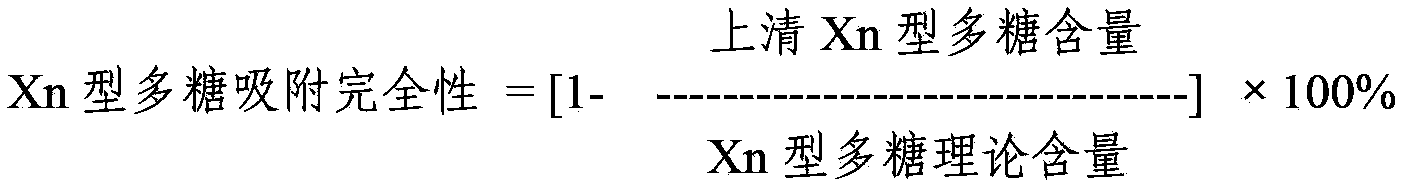
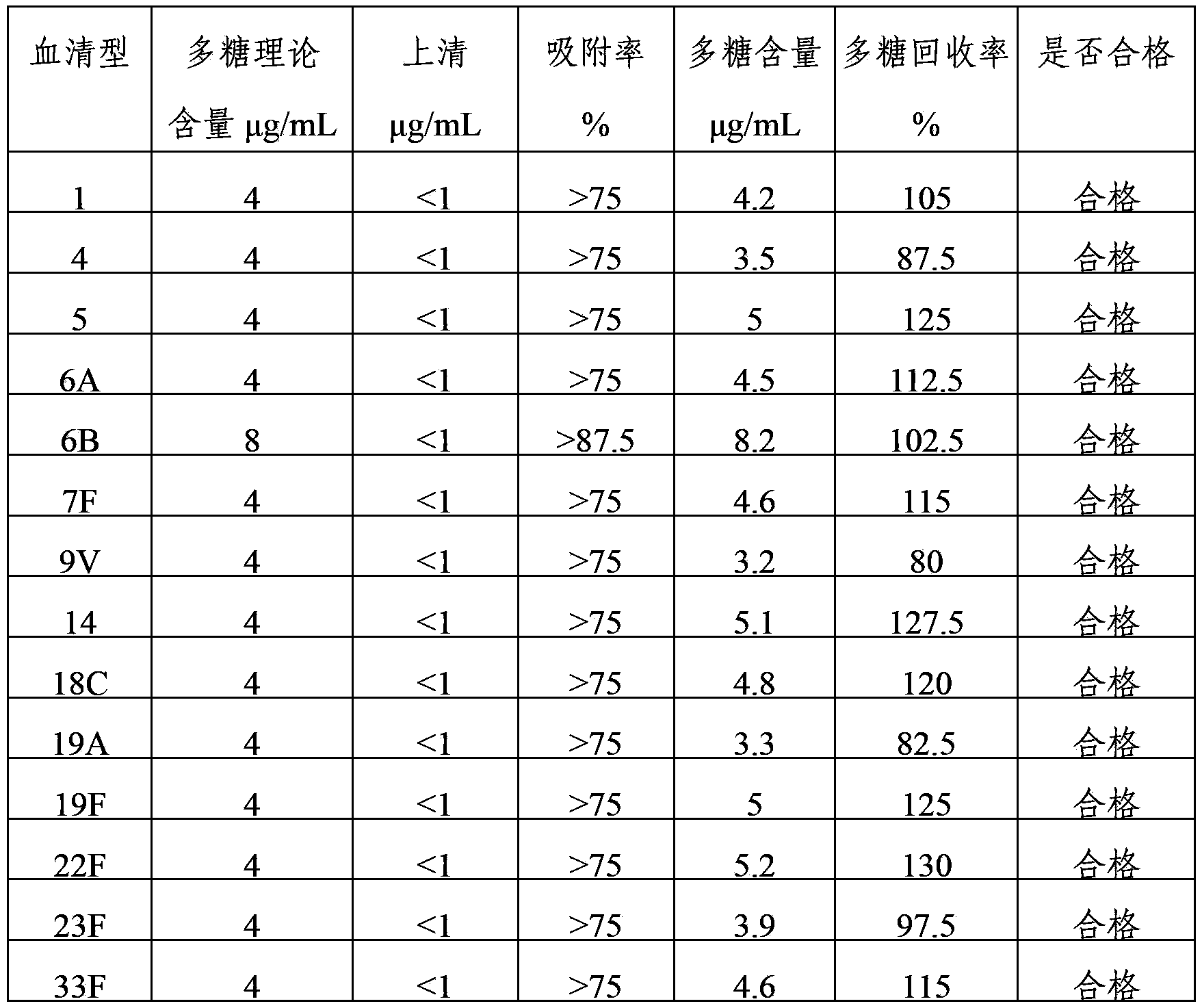
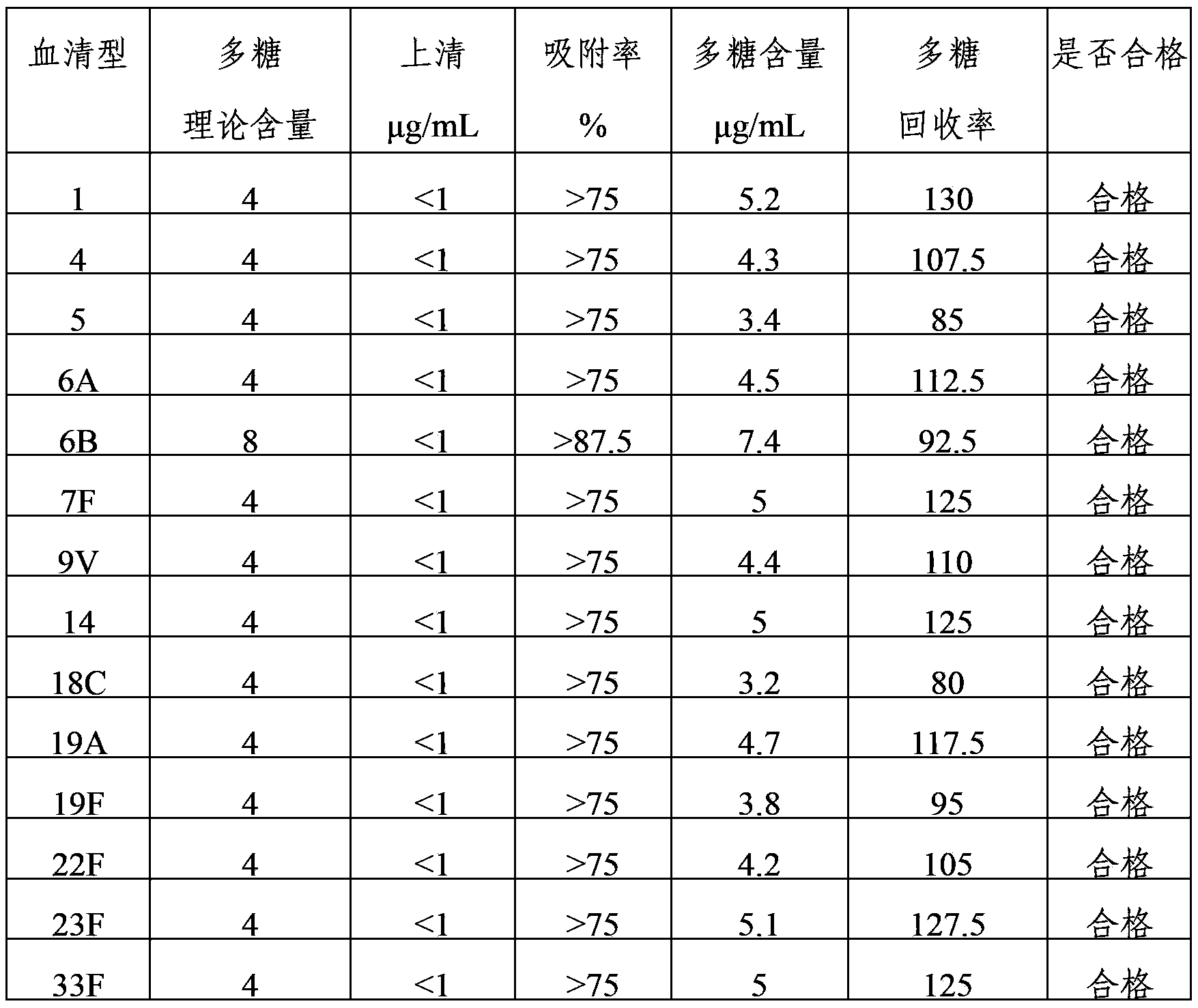
The invention provides a multivalent pneumococcus capsular polysaccharide-protein conjugated composition and a preparation method thereof. The conjugated composition is formed by covalent linkage of multivalent pneumococcus capsular polysaccharides of 14 different serotypes and carrier protein, wherein the 14 serotypes include 1, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F and 33F. The conjugated composition has good adsorption effect and good stability, has multiple immunogenicity and protective performance against invasion of the pneumococcus of 14 serotypes, is superior to on-sale low-valent pneumonia compositions, and the immune response of the conjugated composition disclosed by the invention is higher than that of an uncombined composition. The inoculating injection frequency can be reduced by using the multivalent pneumococcus capsular polysaccharide conjugate vaccine containing the conjugated composition, the immune process can be simplified, and diseases of human and animals caused by the 14 serotypes of pneumococcal bacteria can be effectively prevented. The conjugated composition has wider coverage and better immune effect.
Owner:SINOVAC RES & DEV
Zika-virus-and-yellow-fever-virus combined inactivated vaccine
ActiveCN107537029AStable physical and chemical propertiesReduce the number of vaccinationsViral antigen ingredientsInactivation/attenuationDiseaseZika virus
The invention provides a Zika-virus-and-yellow-fever-virus combined inactivated vaccine, and belongs to the technical field of biological product preparing. 0.5 microgram / ml-10 microgram / ml of yellowfever viruses and 0.5 microgram / ml-10 microgram / ml of Zika viruses are contained in the combined inactivated vaccine. The invention also provides a preparing method for the Zika-virus-and-yellow-fever-virus combined inactivated vaccine; the preparing method includes the steps of Zika-virus and yellow-fever-virus inoculating, purifying and inactivating, wherein yellow-fever-virus inoculated MOI is0.01 PFU / ml to 1 PFU / ml, and Zika-virus inoculated MOI is 0.001 CCID<50> / ml to 0.1 CCID<50> / ml. By means of the Zika-virus-and-yellow-fever-virus combined inactivated vaccine, the Zika viruses and theyellow fever viruses can be immunized at the same time, infection and anaphylaxis which are caused by an attenuated vaccine are avoided, and the Zika-virus-and-yellow-fever-virus combined inactivatedvaccine has the good capacity for controlling diseases caused by the Zika viruses and the yellow fever viruses.
Owner:SINOVAC BIOTECH
Trivalent inactivated vaccine of porcine reproductive and respiratory syndrome virus, porcine circovirus type 2, and porcine pseudorabies virus and preparation method thereof
InactiveCN102973932AIncrease productionImprove securityViral antigen ingredientsAntiviralsImmune effectsMultivalent Vaccine
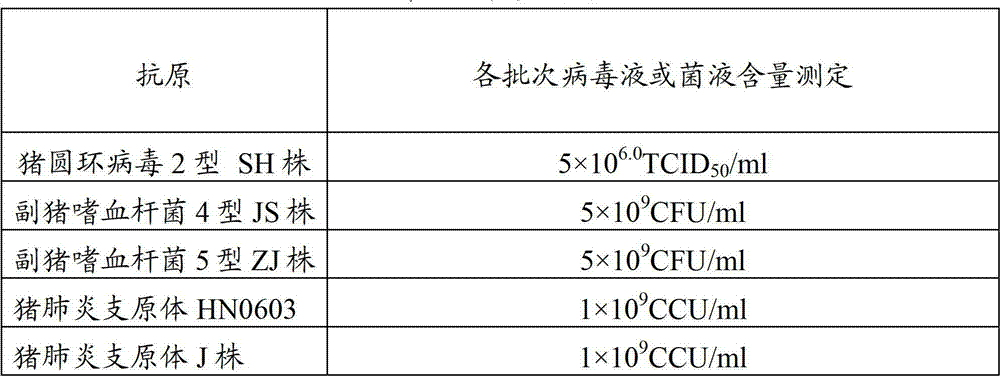
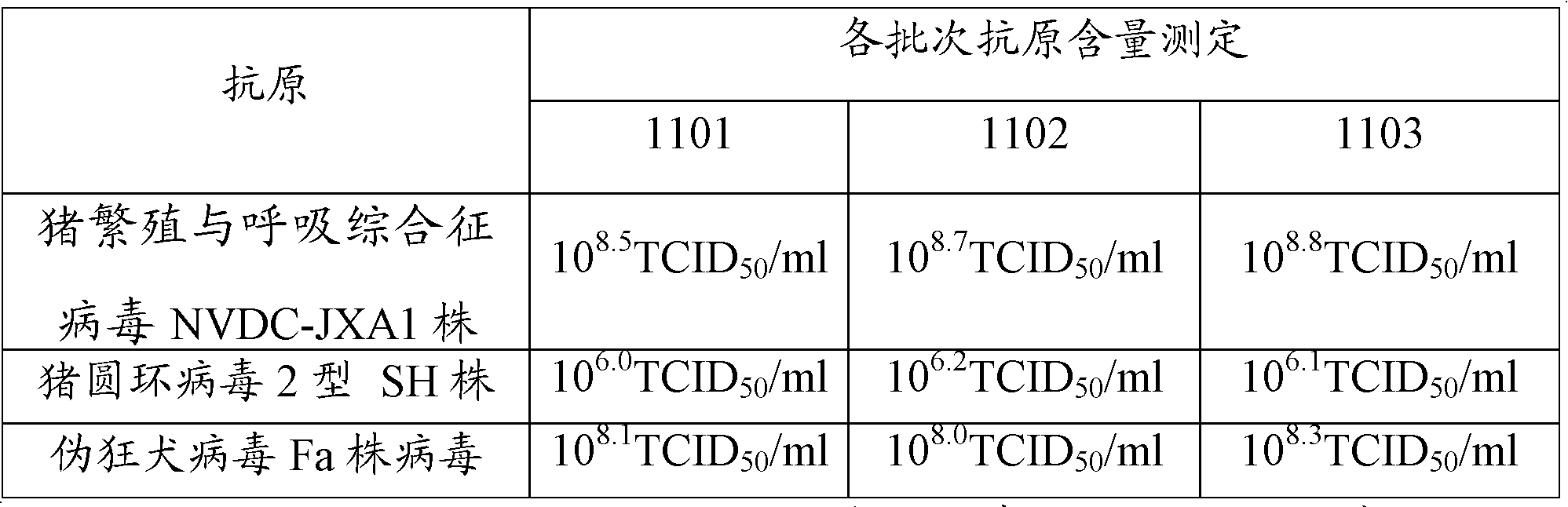
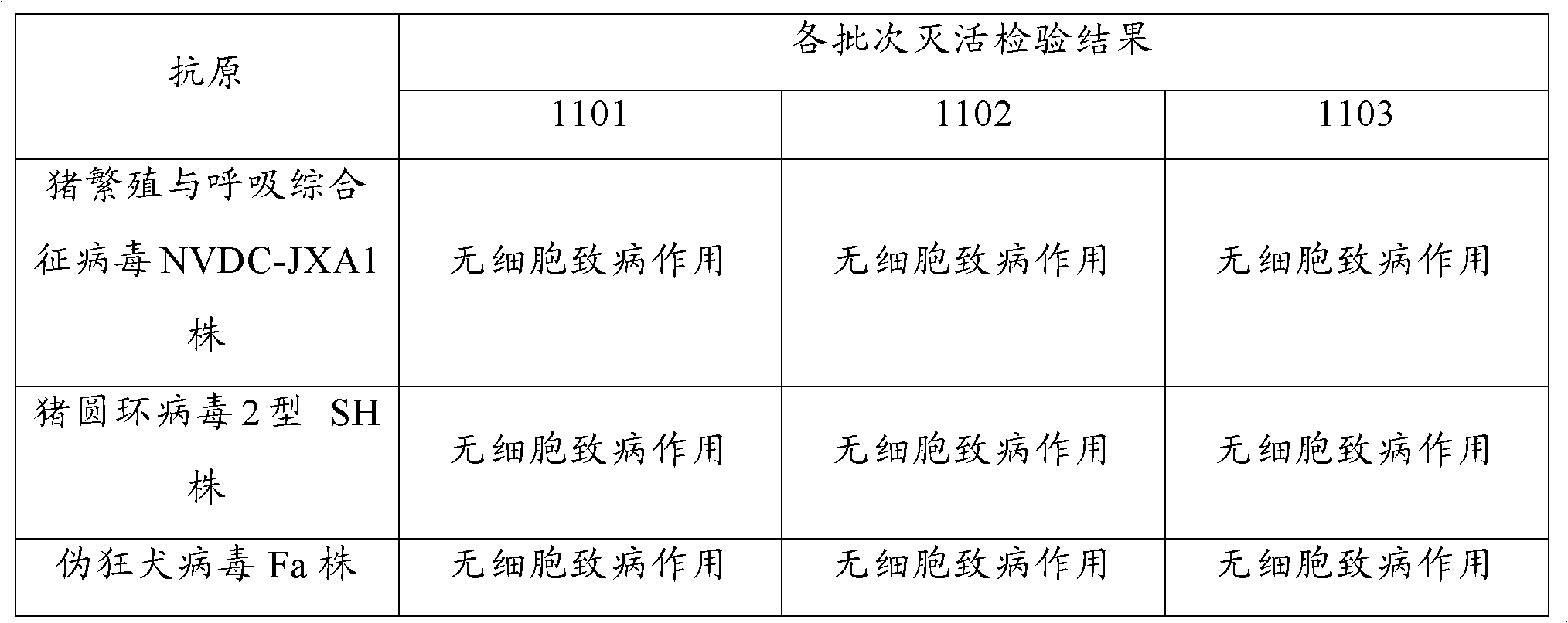
The present invention provides a trivalent inactivated vaccine of porcine reproductive and respiratory syndrome virus, porcine circovirus type 2, and porcine pseudorabies virus. Before inactivation, the contents of the three viruses are respectively greater than 10<8.5>TCID[50] / ml 10<6.0>TCID[50] / ml and 10<8.0>TCID[50] / ml; And after the inactivation, the volume ratio of the three antigens is 1:1:1. According to the present invention, via a large number of detailed tests, the contents and ratio of the three viral antigens are selected; and immune effects are measured in a large number of experimental animals and swine themselves, to ensure that the phenomenon of immune interference does not occur among the various immune components in the multivalent vaccine. Compared with the existing three individual vaccines of the same three virus, wherein three injections are need to prevent the three diseases caused by the three virus by using the three individual vaccines, the trivalent inactivated vaccine of the present invention is economical and practical, and simplifies the immunization procedure, and reduces the cost of epidemic prevention. The present invention realizes preparation and application of multivalent inactivated vaccine of porcine reproductive and respiratory syndrome virus, porcine circovirus type 2, and porcine pseudorabies virus, which has not been achieved for all the time in the field.
Owner:PU LIKE BIO ENG
Polyvalent pneumococcal capsular polysaccharide-protein conjugate composition and preparation method thereof
ActiveCN103656631BImprove adsorption capacityImprove stabilityAntibacterial agentsBacterial antigen ingredientsDiseaseImmune effects
The invention provides a multivalence pneumococcus capsular polysaccharide-protein conjugate composition and a preparation method thereof. The conjugate composition is prepared from capsular polysaccharides of pneumococcus of 24 different serotypes and a carrier protein in a covalence connection manner, wherein the 24 different serotypes are 1, 2, 3, 4, 5, 6A, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F. The conjugate composition is good in adsorption effect and stability, has multiple immunogenicity and protection properties aiming at invasion of pneumococcus of the 24 serotypes, and is superior to the low-valence pneumonia composition in the market, and the immune response is higher than that of uncombined compositions. By inoculating a multivalence pneumococcus capsular polysaccharide conjugate vaccine prepared from the conjugate composition, the inoculation injection times can be reduced, the immunization procedure can be simplified, and meanwhile human beings and animals can be effectively prevented from diseases resulted from the pneumococcus of the 24 serotypes, and the conjugate composition is wide in coverage range and good in immune effect.
Owner:SINOVAC RES & DEV
Recombinant chicken Marek's disease virus transfer vector and application thereof
InactiveCN1763205ALow costSimplified immunization programViral antigen ingredientsMicroorganism based processesMultiple cloning siteViral gene
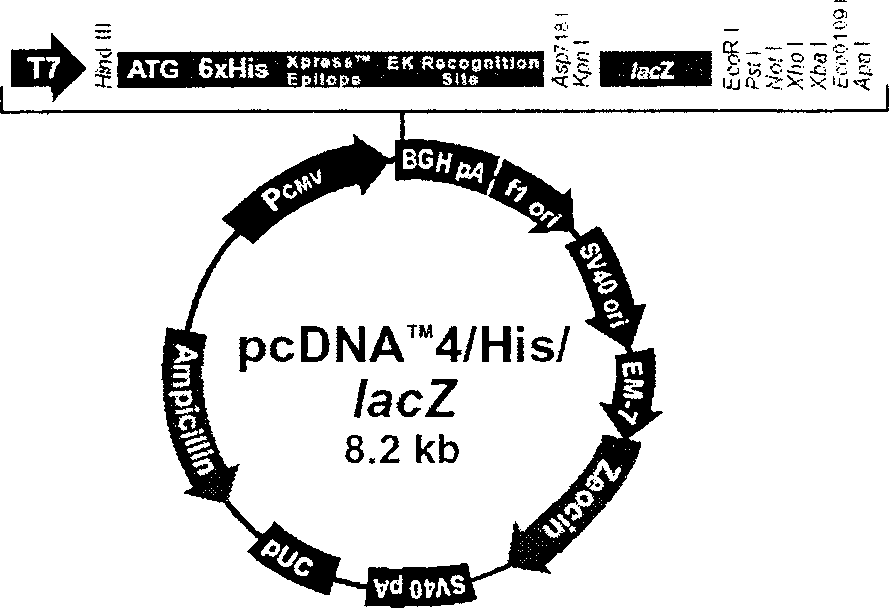
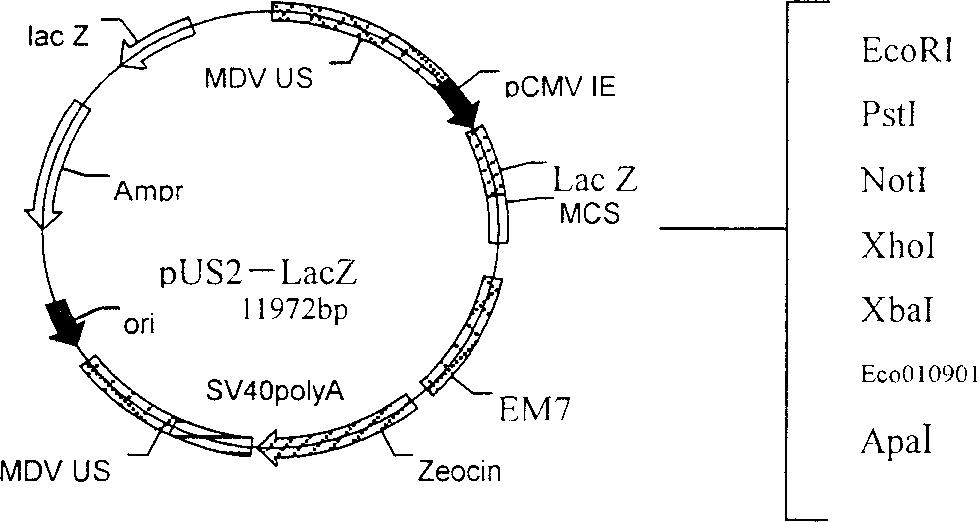
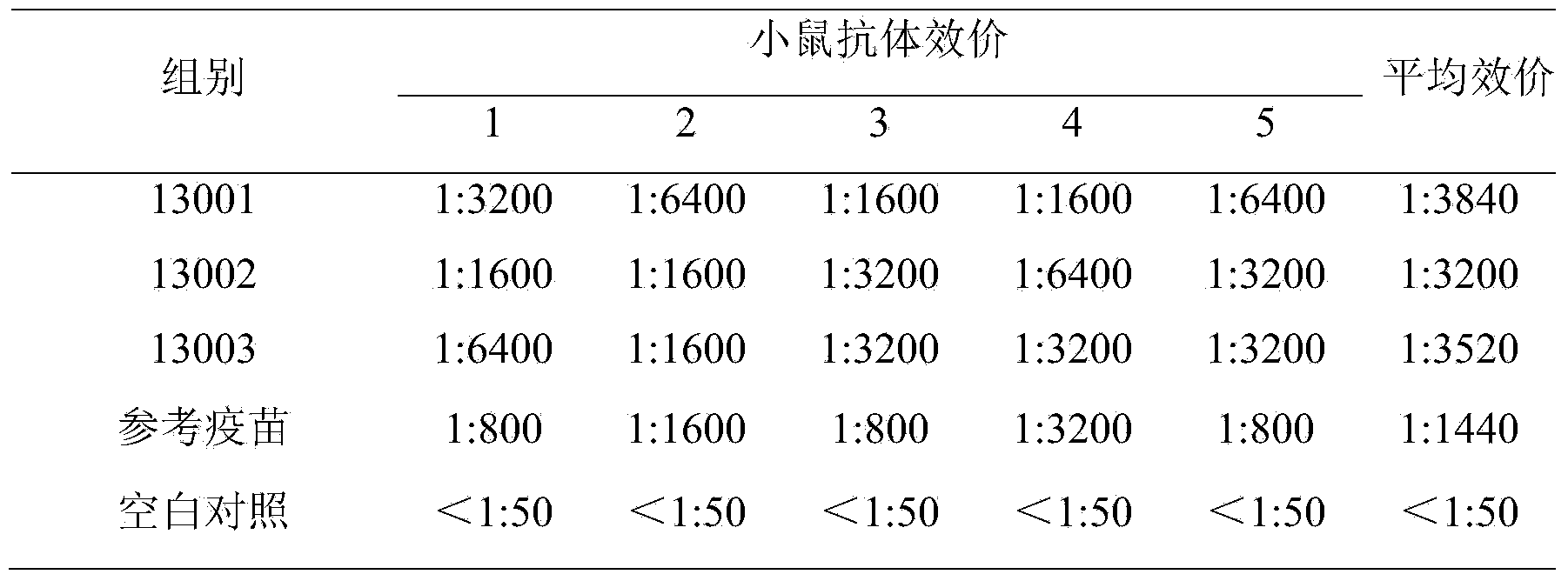
The present invention relates to recombinant chicken Marek's disease virus transfer vector and its application. The transfer vector pUS2-LacZ features that the recombinant plasmid pUS2 has US2 gene segment substituted with CMZ immediate early promoter, LacZ gene, transcription termination signal SV40polyA, ampicillin and neomycin marked gene and one polyclonal site gene. The transfer vector may be homogeneously recombined with MDV vaccine virus to obtain recombinant virus MDV of LacZ marked gene, and has polyclonal site capable of inserting foreign virus gene to obtain recombinant plasmid. Through further homogeneous recombination, purification and screening, recombinant virus is obtained, and the recombinant virus may be prepared into vaccine with effect of resisting several kinds of virus.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Porcine circovirus type 2, porcine reproductive and respiratory syndrome bivalent vaccine and preparation method thereof
ActiveCN104056265AEasy to useStrong stress responseViral antigen ingredientsAntiviralsAdjuvantVaccine antigen
The invention relates to a porcine circovirus type 2, porcine reproductive and respiratory syndrome bivalent vaccine and a preparation method thereof. The bivalent vaccine is a lyophilized vaccine, and comprises a porcine circovirus type 2 SH strain inactivated virus and a respiratory syndrome virus R98 strain live virus, while an adjuvant is added into the vaccine diluent, so that the immunizing effect of the vaccine can be further improved. Immune interference does not exist among the bivalent lyophilized vaccine antigens, and the quality inspection of the vaccine finished product can achieve the quality standard of a single vaccine, and the immune protection effect of the vaccine finished product is better than that of a single vaccine; the bivalent vaccine can be used for simplifying the immune procedure, reducing immune cost and reducing the stress reaction of an immunized pig, and thus the bivalent vaccine has great market application values.
Owner:JIANGSU NANNONG HI TECH
Preparation and application of GIT fusion protein used for preventing dairy cow mastitis
InactiveCN102731660AImproving immunogenicityHigh expressionAntibacterial agentsBacterial antigen ingredientsNucleotideStaphylococcus aureus
The invention provides a GIT fusion protein used for preventing dairy cow mastitis. The GIT fusion protein has an amino acid sequence as represented by SEQ ID No. 1 or an amino acid sequence which is formed after substitution, deletion or addition of one or a plurality of amino acids of the sequence represented by SEQ ID No. 1 and has same functions as the sequence represented by SEQ ID No. 1 does. The invention also provides the nucleotide sequence of the protein and a preparation method and application for the same. The protein has good immunogenicity, can effectively prevent infection caused by Staphylococcus aureus, Streptococcus dysgalactiae, Streptococcus agalactiae and Streptococcus uberis, has a wide immune range and can be used for preventing dairy cow mastitis; moreover, while immunoprotection effects of the GIT fusion protein provided in the invention is guaranteed, preparation process and immune procedures in actual production are simplified, the length of the peptide chain of the fusion protein is shortened, expression of the fusion protein is enhanced, and the GIT fusion protein has an important value in development and application of novel vaccines.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Anti-porcine pseudorabies and swine flu vaccine composition and application thereof
ActiveCN103893750AImprove immunityNo interferenceAntiviralsAntibody medical ingredientsDiseaseAntigen
The invention relates to an anti-porcine pseudorabies (PRV) and swine flu (SIV) vaccine composition. Besides porcine pseudorabies and swine flu, the composition can additionally prevent clinical manifestations of porcine respiratory disease complex (PRDC) caused by infection of other pathogenic causes. Furthermore, the invention provides application of the vaccine composition for preparing a medicine in preventing and / or controlling immunized pig PRDC disease. The composition not only avoids interference between antigens in a process of using but also achieves a surprising preventive effect on the porcine respiratory disease complex. Therefore, the vaccine composition disclosed by the invention can simplify an immune procedure and achieve an effect of preventing two diseases by one dosage. The anti-porcine pseudorabies and swine flu vaccine composition, after immunizing pigs, can effectively prevent spread of the PRDC.
Owner:PU LIKE BIO ENG
Preparation method for newcastle disease and infectious bursal disease bigeminal composite inactivated vaccine
ActiveCN103585626BImprove immunityImprove ability to prevent diseaseViral antigen ingredientsAntiviralsAntigenDisease
Owner:浙江美保龙生物技术有限公司
Mycoplasma hyorhinis strain, vaccine composition, preparation method and application thereof
ActiveCN104250623AImprove the effect of prevention and controlImmunity overAntibacterial agentsBacterial antigen ingredientsImmune effectsMycoplasma
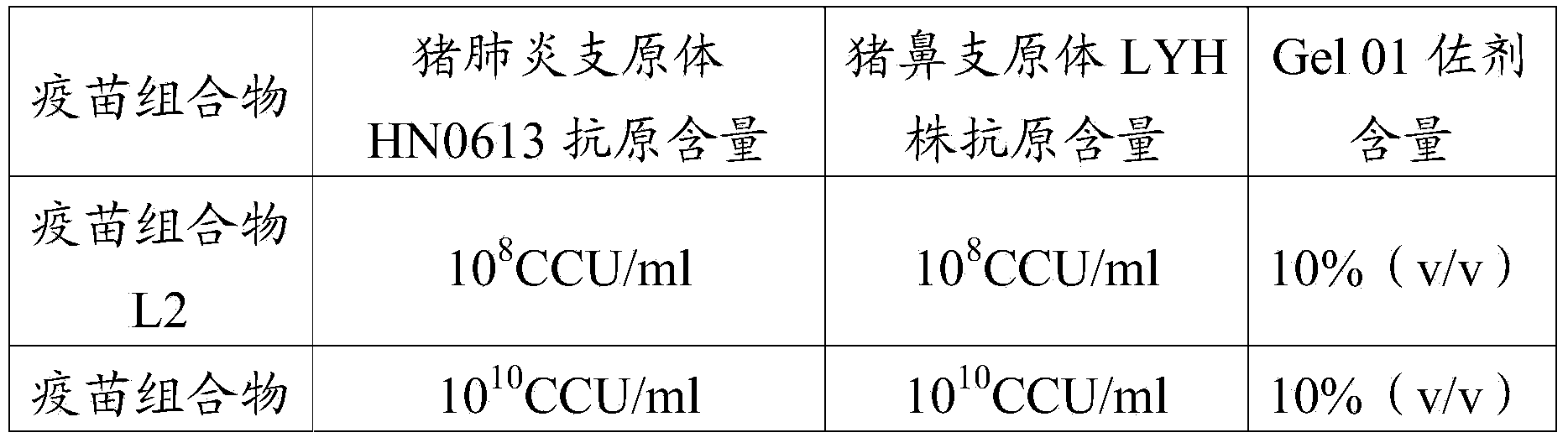
The invention provides a Mycoplasma hyorhinis strain LYH, and a vaccine composition prepared from the Mycoplasma hyorhinis strain LYH, in particular to a vaccine composition comprising the Mycoplasma hyorhinis and Mycoplasma hyopneumoniae. The vaccine composition can be effective in prevention and treatment of swine enzootic pneumonia caused by Mycoplasma hyorhinis, Mycoplasma hyopneumoniae single infection or mixed infection. Especially in the circumstance of mixed infection, immune effect of the vaccine composition significantly exceeds that of each single vaccine.
Owner:PU LIKE BIO ENG
Vaccine vector preventing FAdV-4 and NDV and preparation method and application of vaccine vector
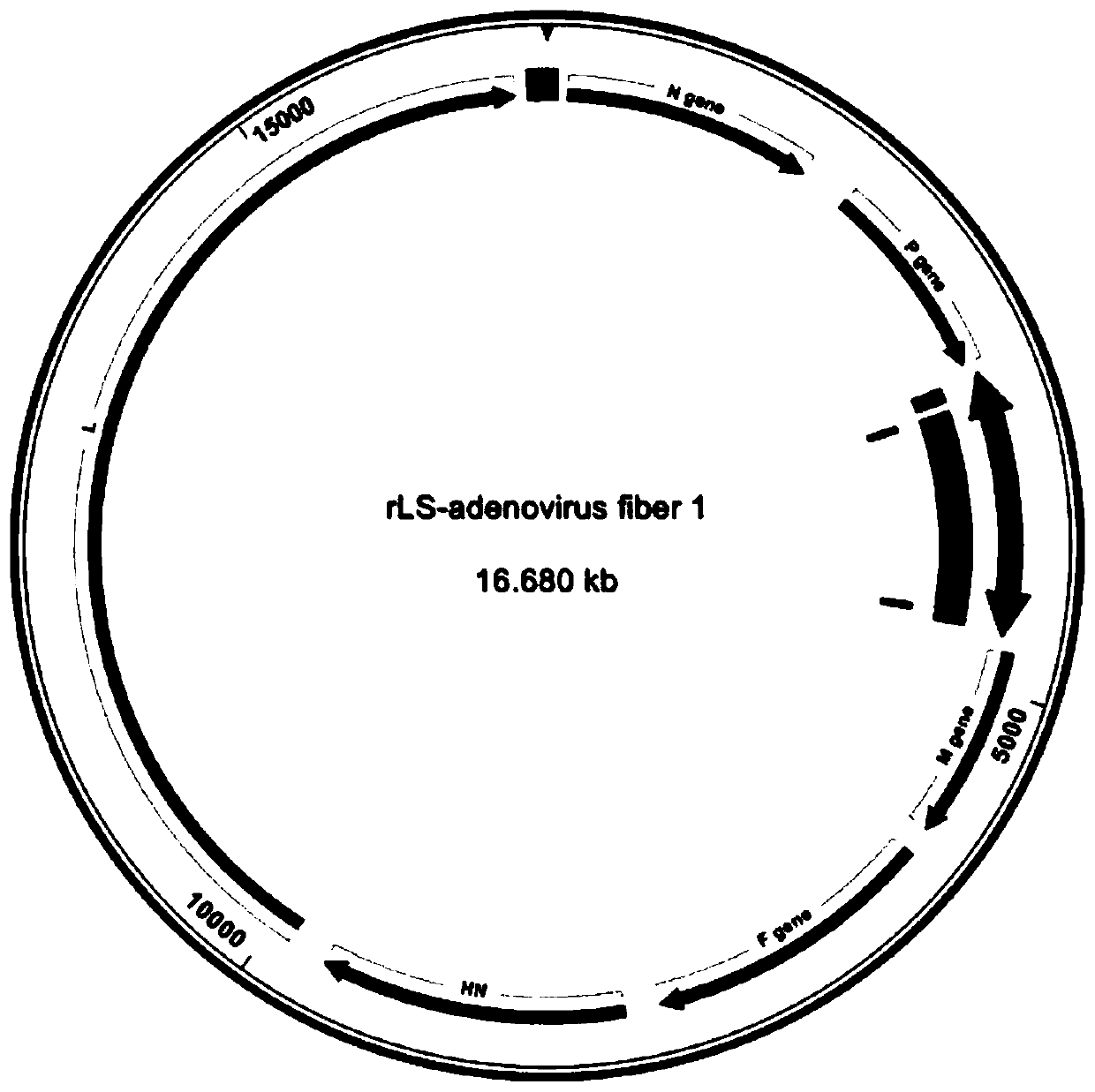
ActiveCN110484515ASimplified immunization programGood immune protectionSsRNA viruses negative-senseViral antigen ingredientsFiberProtective antigen
The invention relates to a vaccine vector preventing the FAdV-4 and the NDV and a preparation method and application of the vaccine vector, and belongs to the technical field of prevention for the FAdV-4 and the NDV. According to vaccine vector and the preparation method and application of the vaccine vector, based on the structure characteristic of the FAdV-4, an FAdV-4 protective antigen Fiber 1protein is selected as a research and development object, and by constructing a recombinant Newcastle disease virus expressing the Fiber 1, a novel genetic engineering vaccine capable of preventing the FAdV-4 and the NDV is obtained through research and development; through research and development of the vaccine, not only is an important tool provided for prevention and control over the FAdV-4,but also a LaSota toxic strain of the Newcastle disease virus is selected as the vaccine vector, the toxic strain is a common vaccine toxic strain in production, an effect can be achieved that one strain prevents the two viruses, and therefore the vaccine immunization process is simplified.
Owner:HENAN UNIV OF SCI & TECH
Vaccine composition for resisting pig mycoplasma pneumonia and infectious pleuropneumonia and preparation method
ActiveCN103623400AReduce harmSimplified immunization programAntibacterial agentsBacterial antigen ingredientsTGE VACCINEMycoplasma pneumonia
The invention relates to a vaccine composition for resisting pig mycoplasma pneumonia and infectious pleuropneumonia and a preparation method. The vaccine composition comprises pig mycoplasma hyopneumoniae antigens and pig actinobacillus pleuropneumoniae antigens, is simple in immunization procedure, not only is capable of generating antibodies, but also has the functions of toxicity attack protection and effectively controlling pig mycoplasma pneumonia and infectious pleuropneumonia. The vaccine composition has the immunization effect same to an independently injected vaccine, is small in side reaction, long in immunization period, less in consumed time, less in consumed labor, small in encroaching on a pig. The vaccine composition is simple in production technology, low in immunization cost and strong in practicality.
Owner:PU LIKE BIO ENG
Pig pseudorabies live vaccine-pig mycoplasma pneumonia live vaccine composition and application thereof
ActiveCN105288612ASolve the problem of continuous stressAvoid problems with risk of potency dropAntibacterial agentsBacterial antigen ingredientsPig farmsImmune effects
The invention relates to a pig pseudorabies live vaccine-pig mycoplasma pneumonia live vaccine composition and the application thereof. When the vaccine composition is applied to immune animals, two vaccines can be obtained to achieve different immune effects, continuous stress on inoculated animals caused by independent immunity can be avoided, immune procedures are simplified, and immunization workload is reduced for a pig farm.
Owner:QILU ANIMAL HEALTH PROD
Polyvalent pneumococcal capsular polysaccharide composition, its preparation method and application
ActiveCN103656632BStable physical and chemical propertiesPrevent diseaseAntibacterial agentsBacterial antigen ingredientsConjugate vaccineStreptococcus pneumoniae capsular polysaccharide
The invention provides a multivalent pneumococcal capsular polysaccharide composition as well as a preparation method and application thereof. The multivalent pneumococcal capsular polysaccharide composition contains a serotype 6A and at least one extra serotype selected from the group consisting of 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F. The multivalent pneumococcal capsular polysaccharide composition provided by the invention can be used for inducing an organism to generate humoral immunity, can generate a relatively good protecting effect for infectious diseases caused by the 24 common serotype pneumococcuses and is wide in immunity coverage rate and better in effect as comparison with various existing pneumococcal polysaccharide vaccines and conjugate vaccines sold on the market.
Owner:SINOVAC RES & DEV
Tilmicosin injection as well as preparation method and application thereof
ActiveCN105456184ASmall toxicityExpand the scope of clinical applicationAntibacterial agentsSsRNA viruses negative-senseAstragalosideAntioxidant
The invention provides tilmicosin injection as well as a preparation method and an application thereof. The tilmicosin injection is prepared from the following components in parts by weight: 10-50 parts of tilmicosin, 1-5 parts of astragaloside, 0.5-2 parts of ATP, 5-20 parts of a cosolvent, 0.1-0.5 part of an antioxidant, and 15-30 parts of a stabilizer. The tilmicosin injection is stable in property, remarkable in effect, and small in irritation and safe to the injected part, and can be mixed with inactivated vaccines for injection, so that the clinic application of the tilmicosin injection is expanded.
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
Method for preparing inactivated vaccine both for preventing chicken Newcastle disease and infectious bronchitis
InactiveCN101099864BImproving immunogenicityStrong specificityViral antigen ingredientsRespiratory disorderInfectious bronchitisEmulsion
The invention is concerned with a kind of bacterin to prevent fowl infectious disease, relating to a kind of bacterin preparation method to prevent Newcastle Disease and infectious bronchitis. The selected genus is Newcastle Disease virus La Sota individual plant, infectious bronchitis virus M41 individual plant and HN99 kidney type of aberrance individual plant. Dilute two kind of virus and inoculate in chick embryo allantoic cavity, collect liquid of embryo to alive and dead embryos as seed. Dilute and inoculate in allantoic cavity of affectable chick embryo to get liquid of virus embryo. Concentrate with equipment and add with formaldehyde solution to prepare bacterin through emulsion process. This invention can prevent Newcastle Disease, chick kidney type and breathing type infectiousbronchitis, and it can reduce the times of injection and the cost of epidemic prevention for easy using and practicality.
Owner:HENAN AGRICULTURAL UNIVERSITY
Porcine circovirus, porcine pseudorabies virus and mycoplasma triple inactivated vaccine
PendingCN112957460AReduce the chance of side effectsHigh antigen contentAntibacterial agentsBacterial antigen ingredientsUltrafiltrationVirus Protein
The invention discloses a porcine circovirus, porcine pseudorabies virus and mycoplasma triple inactivated vaccine which comprises an antigen and a vaccine adjuvant, the antigen is composed of a porcine circovirus type 2 antigen, a porcine pseudorabies virus antigen and a mycoplasma antigen, the porcine circovirus type 2 antigen is a purified, concentrated and inactivated porcine circovirus type 2 protein antigen solution, and the content of Cap protein is more than or equal to 160 [mu]g / ml; the porcine pseudorabies virus antigen is a purified, concentrated and inactivated porcine pseudorabies virus protein antigen solution, and the content of the Cap protein is more than or equal to 160 [mu]g / ml; the mycoplasma antigen is an inactivated mycoplasma protein antigen solution, and the content of the Cap protein is more than or equal to 160 [mu]g / ml; and the vaccine adjuvant is composed of a water-based high-molecular polymer adjuvant and a composite polysaccharide immunopotentiator. Foreign protein is removed through clarification filtration and ultrafiltration concentration, and the side reaction probability of the vaccine is greatly reduced; and three-proofing can be achieved through one needle, so that the number of immunization times and stress are reduced. The method is economical and practical, the immunization procedure is simplified, and the epidemic prevention cost is reduced.
Owner:JIANGXI ZHENGBANG TECHNOLOGY CO LTD +1
Immune combined preparation and preparation method and application thereof
PendingCN110251667APrevent diseaseWide coverageAntibacterial agentsBacterial antigen ingredientsBacteroidesImmune effects
The invention discloses an immune combined preparation and a preparation method and an application thereof, wherein the immune combined preparation comprises a streptococcus pneumoniae capsular polysaccharide-CRM197 protein conjugate and a haemophilus influenzae B capsular polysaccharide-CRM197 protein conjugate, wherein the mass ratio of a haemophilus influenzae B capsular polysaccharide to a CRM1197 protein is (0.3-1):1. The mass content of the haemophilus influenzae type B capsular polysaccharide is 10-15 [mu]g / dose. The combined vaccine can effectively induce a body to produce high-titer and specific antibodies against the selected serotype streptococcus pneumoniae polysaccharide and haemophilus influenzae type B capsular polysaccharide; and no interference exists between antigen components, and higher immune effect is achieved by screening the combination way; after the vaccine is prepared, the number of vaccinations can be reduced, the immune procedure is simplified, and human and animal diseases caused by the bacteria are effectively prevented.
Owner:BRAVOVAX
A combined inactivated vaccine of Zika virus and yellow fever virus
ActiveCN107537029BStable physical and chemical propertiesReduce the number of vaccinationsViral antigen ingredientsInactivation/attenuationDiseaseZika virus
The invention provides a Zika-virus-and-yellow-fever-virus combined inactivated vaccine, and belongs to the technical field of biological product preparing. 0.5 microgram / ml-10 microgram / ml of yellowfever viruses and 0.5 microgram / ml-10 microgram / ml of Zika viruses are contained in the combined inactivated vaccine. The invention also provides a preparing method for the Zika-virus-and-yellow-fever-virus combined inactivated vaccine; the preparing method includes the steps of Zika-virus and yellow-fever-virus inoculating, purifying and inactivating, wherein yellow-fever-virus inoculated MOI is0.01 PFU / ml to 1 PFU / ml, and Zika-virus inoculated MOI is 0.001 CCID<50> / ml to 0.1 CCID<50> / ml. By means of the Zika-virus-and-yellow-fever-virus combined inactivated vaccine, the Zika viruses and theyellow fever viruses can be immunized at the same time, infection and anaphylaxis which are caused by an attenuated vaccine are avoided, and the Zika-virus-and-yellow-fever-virus combined inactivatedvaccine has the good capacity for controlling diseases caused by the Zika viruses and the yellow fever viruses.
Owner:SINOVAC BIOTECH
Vaccine composition for preventing and treating porcine respiratory syndrome, and preparation method and application thereof
ActiveCN103961695ALow costReduce the number of vaccinationsAntibacterial agentsAntiviralsHaemophilusMedicine
A provided vaccine composition for preventing and treating porcine respiratory syndrome comprises immunization amount of porcine influenza virus antigen, immunization amount of porcine pneumonia mycoplasma antigen, immunization amount of haemophilus parasuis antigen, and an adjuvant. The vaccine composition is capable of effectively preventing and controlling porcine respiratory syndrome caused by mixed infection of the three kinds of pathogeny, and also is capable of reducing vaccine inoculating frequency and avoiding unavailable full-access immunization caused by missed inoculation.
Owner:PU LIKE BIO ENG
Novel flow method adeno-quadruplet virus-like particle and preparation method and application thereof
ActiveCN112458118BSimplified immunization programAvoid mutual interferenceSsRNA viruses negative-senseViral antigen ingredientsVirus-like particleViral nucleic acid
A new flow method adeno-quadruplet virus-like particle and its preparation method and application belong to the field of quadruple vaccine research and development. Rescue, preparation and purification of chimeric virus-like particles, and finally obtain chimeric virus-like particles NDV‑AIV‑IBDV‑FAdV4 cVLPs. The new flow method AAV-like particle prepared by the preparation method of the present invention has the following advantages: the new flow method AAV-like particle can repeatedly display foreign antigens with high density, and has strong immunogenicity; It does not contain viral nucleic acid, green and safe, which is conducive to the purification of new flow glands, and conforms to the modern green and safe breeding concept; the vaccine seed virus is matched with the popular virus strain; one shot for multiple prevention, simplifying the vaccine immunization procedure, avoiding a variety of Mutual interference of vaccines; suitable for large-scale suspension culture and mass production.
Owner:JILIN UNIV
Vaccine composition and preparation method against swine mycoplasma pneumonia and infectious pleuropneumonia
ActiveCN103623400BReduce harmSimplified immunization programAntibacterial agentsBacterial antigen ingredientsSingle injectionSwine Mycoplasma Pneumonia
The invention relates to a vaccine composition against swine mycoplasma pneumonia and infectious pleuropneumonia and a preparation method thereof. The vaccine composition contains Mycoplasma hyopneumoniae antigen and Actinobacillus pleuropneumoniae antigen, has simple immunization procedures, can produce antibodies, and has protection against viruses at the same time, and can effectively prevent and treat Mycoplasma pneumoniae pneumonia and porcine infectious pleuropneumonia. The immune effect of the vaccine composition is at least equal to the effect of separate injection of single vaccine, less side effects, high serum antibody titer, long immunization period, less time-consuming, less labor, and less damage to pigs. The vaccine composition has simple production process, low immunization cost and strong practicability.
Owner:PU LIKE BIO ENG
Bovine infectious rhinotracheitis virus and mycoplasma bovis dual inactivated vaccine and its preparation method and suspension mdbk cells used
ActiveCN111671893BGuaranteed stabilityQuality improvementAntibacterial agentsBacterial antigen ingredientsAntigenDisease
Owner:CHINA AGRI UNIV
Vaccine composition for preventing and treating porcine circovirus type 2, Haemophilus parasuis and Mycoplasma hyopneumoniae infection and preparation method thereof
ActiveCN103083655BSimplified immunization programReduce manufacturing costAntibacterial agentsBacterial antigen ingredientsDiseaseCircovirus
The invention provides a vaccine composition for preventing and treating porcine circovirus type 2, haemophilus parasuis and mycoplasma hyopneumoniae infection. The vaccine composition comprises an inactivated porcine circovirus type 2 antigen, inactivated haemophilus parasuis, inactivated mycoplasma hyopneumoniae and a vaccine adjuvant. The vaccine composition disclosed by the invention can realize the aim of preventing three diseases including a porcine circovirus disease, mycoplasma pneumonia, a haemophilus parasuis disease by one injection of the vaccine; the content of antigen is 1 / 2 of the content of a common single-vaccine antigen when the vaccine composition disclosed by the invention is prepared by mixing the three antigens; and compared with the existing condition that three injections of single vaccine are injected to prevent three infectious diseases, the technical scheme disclosed by the invention is economical and practical, reduces the production cost, simplifies an immune procedure and reduces the epidemic prevention cost.
Owner:PU LIKE BIO ENG
A vaccine composition for preventing and treating porcine respiratory syndrome, its preparation method and application
ActiveCN103961695BLow costReduce the number of vaccinationsAntibacterial agentsAntiviralsHaemophilusMycoplasma antigen
A provided vaccine composition for preventing and treating porcine respiratory syndrome comprises immunization amount of porcine influenza virus antigen, immunization amount of porcine pneumonia mycoplasma antigen, immunization amount of haemophilus parasuis antigen, and an adjuvant. The vaccine composition is capable of effectively preventing and controlling porcine respiratory syndrome caused by mixed infection of the three kinds of pathogeny, and also is capable of reducing vaccine inoculating frequency and avoiding unavailable full-access immunization caused by missed inoculation.
Owner:PULIKE BIOLOGICAL ENG INC
Triple inactivated vaccine for porcine epidemic diarrhea, porcine transmissible gastroenteritis and porcine deltacoronavirus and preparation method thereof
InactiveCN107899007BImmunization method is simpleAddressing the absence of vaccinesSsRNA viruses positive-senseViral antigen ingredientsDiseaseMultivalent Vaccine
The invention provides a triple inactivated vaccine for porcine epidemic diarrhea (PED), porcine transmissible gastroenteritis (TGE) and porcine delta coronavirus (PDCoV) and a preparation method of the triple inactivated vaccine, before inactivation, the contents of the three viruses are greater than or equal to 10<7.0>TCID50 / mL, and after inactivation, the volume ratio of the antigens is 1 to 1to 1. With the triple inactivated vaccine disclosed by the invention, the problem that an effective multivalent vaccine for preventing and treating three diseases including porcine epidemic diarrhea (PED), porcine transmissible gastroenteritis (TGE) and porcine delta coronavirus (PDCoV) is not available on the market is solved, especially, the problem that the vaccine for the porcine delta coronavirus (PDCoV) epidemic in recent years is not available is solved. The triple inactivated vaccine provided by the invention is economical and practical, the immunizing procedure is simplified, the epidemic prevention cost can be effectively reduced, and a novel method for simultaneously preventing the occurrence of the three diseases is provided for domestic breeding enterprises.
Owner:YULIN UNIV +1
A kind of porcine pseudorabies live vaccine-mycoplasma swine pneumonia live vaccine combination and its application
ActiveCN105288612BSolve the problem of continuous stressAvoid problems with risk of potency dropAntibacterial agentsBacterial antigen ingredientsImmune effectsPig farms
The invention relates to a pig pseudorabies live vaccine-pig mycoplasma pneumonia live vaccine composition and the application thereof. When the vaccine composition is applied to immune animals, two vaccines can be obtained to achieve different immune effects, continuous stress on inoculated animals caused by independent immunity can be avoided, immune procedures are simplified, and immunization workload is reduced for a pig farm.
Owner:QILU ANIMAL HEALTH PROD
Vaccine composition and preparation method and application thereof
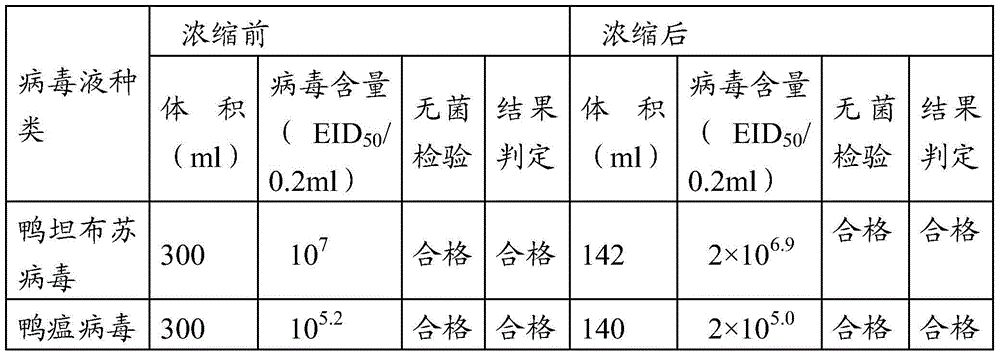
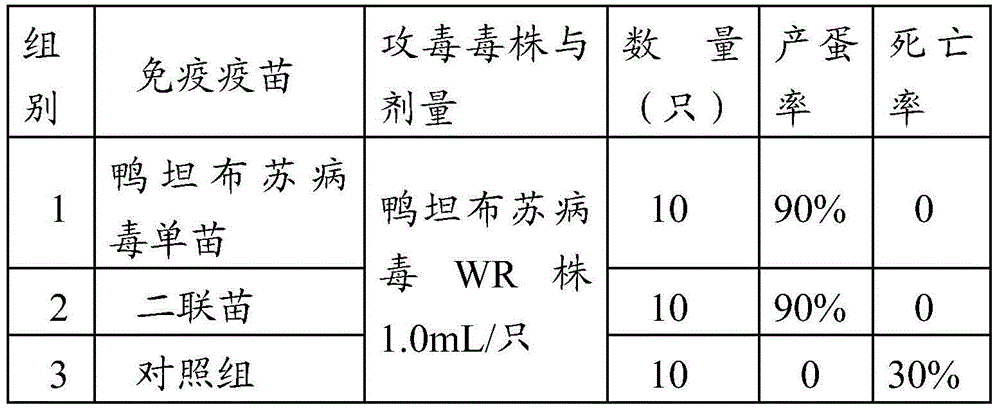
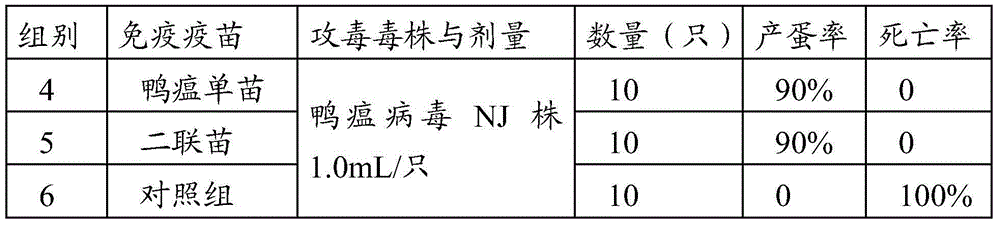
InactiveCN104436186ASimplified immunization programDecrease in egg production rate or even stop productionViral antigen ingredientsAntiviralsDiseaseAntigen
The invention provides a vaccine composition. The vaccine composition comprises an immunization amount of duck tembusu virus antigen, an immunization amount of duck plague virus antigen and one or several adjuvants acceptable in veterinary medicine. The vaccine composition not only can simplify the immunization procedure achieving the purpose of preventing two diseases by injection once, but also can generate protective immunity against duck tembusu virus and duck plague and the phenomena of laying rate decrease or even zero laying rate and mass mortality for a duck group which result from mixed infection of the two diseases can also be avoided.
Owner:PU LIKE BIO ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com