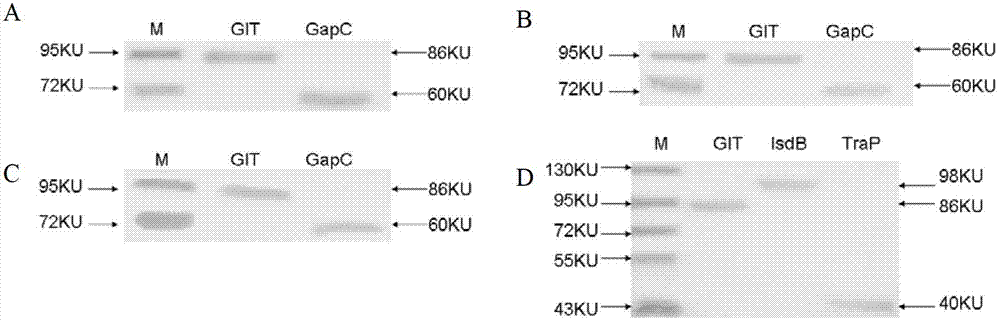
Preparation and application of GIT fusion protein used for preventing dairy cow mastitis
A technology of cow mastitis and fusion protein, which is applied in the direction of medical preparations containing active ingredients, hybrid peptides, and the use of carriers to introduce foreign genetic materials, etc., can solve the problem that the immune protection effect is not as good as that of fusion proteins, and achieve guaranteed Immunoprotective effect, simplified preparation process, shortened peptide chain length
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
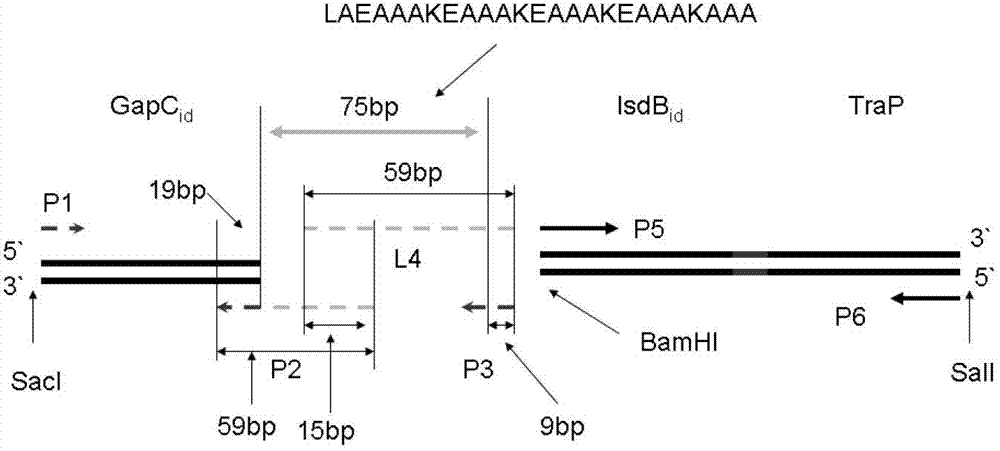
[0045] This example illustrates the construction of intermediate plasmids containing gapC1 and gapC2, the specific steps are as follows:
[0046] 1. According to the published gapC gene sequence, the gene is divided into two fragments, the sequences are shown in SEQ ID No.3 and SEQ ID No.4, and the amino acid sequences of gapC1 and gapC2 are shown in SEQ ID No.5 and SEQ ID As shown in No.6, use Oligo6.0 and Gene Runner software to design two pairs of PCR primers named F1, R1 (for amplifying the gapC1 gene fragment), and F2, R2 (for amplifying the gapC2 gene fragment). The codes, primer positions, sequences and amplified fragment lengths of the primers are listed in Table 1. The upstream primer used to amplify the gapC1 gene fragment introduces a BamH I restriction enzyme site, the downstream primer introduces a Hind III restriction enzyme site, and the upstream primer used to amplify the gapC2 gene fragment introduces a BamH I restriction enzyme The cleavage site, the Sal I r...
Embodiment 2
[0055] This example illustrates the construction of recombinant protein prokaryotic expression vectors gapC-pET32a, gapC1-pET32a, gapC2-pET32a, induction and purification of recombinant protein, and the specific steps are as follows:
[0056] 1. Use BamH I and Sal I to double-digest a part of pET32a for the connection of gapC and gapC2 gene fragments; use restriction endonucleases BamH I and Hind III to double-digest part of the pET-32a plasmid, and use Ligated to the gapC1 gene fragment, pET32a after enzyme digestion was recovered and purified;
[0057] 2. The recombinant cloning plasmid gapC1-pMD18 was double digested with restriction endonucleases BamH I and Hind III, and the recombinant cloning plasmids gapC2-pMD18 and gapC-pQE30 were digested with restriction endonucleases BamH Ⅰ and Sal Ⅰ respectively. Perform double enzyme digestion, and recover and purify the gapC1, gapC2, and gapC gene fragments after enzyme digestion;
[0058] 3. Ligate the gapC1, gapC2, and gapC ge...
Embodiment 3
[0061] This embodiment is used to compare the immunity containing gapC1, gapC2, gapC protein, and is divided into the following parts:
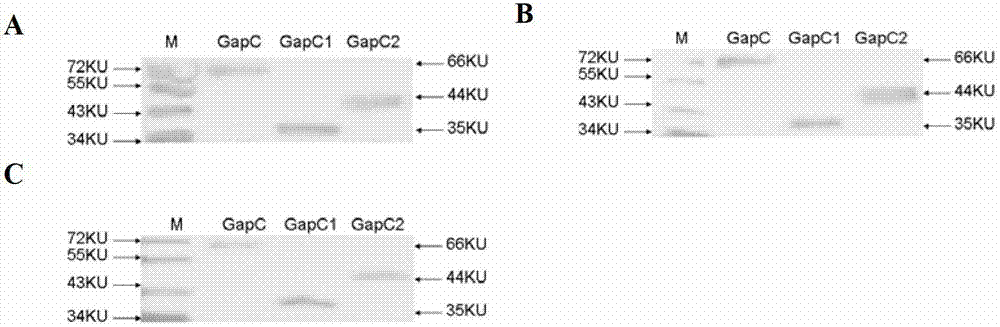
[0062] 1. Use the Western Blotting method to detect the immunogenicity of the recombinant protein (wet transfer method)
[0063] The purified GapC protein, GapC1 protein and GapC2 protein and the pre-stained protein Maker were subjected to Western Blotting respectively according to anti-serum of Streptococcus lactis (1:100), anti-serum of Streptococcus agalactiae (1:200), anti-serum of Streptococcus uberis Dilute the primary antibody with serum (1:100), and dilute the secondary antibody with HRP-labeled goat anti-mouse IgG (1:400in TBST). The result is as Figure 5 As shown, GapC protein, GapC1 protein and GapC2 protein can all react specifically with the antiserum of Streptococcus dysgalactiae, Streptococcus agalactiae, and Streptococcus uberis, and specific bands appear at the corresponding positions of the NC membrane.
[0064] 2. Mice i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com