Multivalent pneumococcal capsular polysaccharide composition as well as preparation method and application thereof
A technology of capsular polysaccharide and pneumococcus, which is applied in the field of microbiology and immunology, can solve the problems of limited protection range and lack of protection of serotype, and achieve the effect of reducing the number of vaccination needles, good protection effect and complete adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of pneumococcal capsular polysaccharide stock solution
[0033] 1, 2, 3, 4, 5, 6A, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F serotype pneumonia The strains of Streptococcus pneumoniae were all obtained from the China Institute for the Control of Pharmaceutical and Biological Products and the Danish Serum Institute (SSI). The above strains were subcultured to establish the original seed bank, main seed bank and working seed bank. The generations were as follows: the original seed batch was the first generation, the main seed batch was the fourth generation, and the working seed batch was the eighth generation. From the opening of the working seed batch to the cultivation in the inoculated fermenter, the passage should not exceed 10 generations. The seeds of each generation use milk powder as a freeze-drying protective agent and freeze-dried for preservation. Take the working seeds and pick the strains by ...
Embodiment 2
[0038] Example 2 Preparation of polyvalent pneumococcal capsular polysaccharide composition
[0039] The 24 kinds of pneumococcal capsular polysaccharide stocks prepared in Example 1 were mixed according to the final concentration of 50 μg / ml polysaccharide content of each serotype, and then buffer solution (water for injection, physiological saline or phosphate buffer) was added to make up to the final volume. Prepare 24-valent pneumococcal polysaccharide composition.
[0040] The 24-valent pneumococcal capsular polysaccharide vaccine prepared above is made into a freeze-dried dosage form by low-temperature drying, and reconstituted with injection water, physiological saline or phosphate buffer before use.
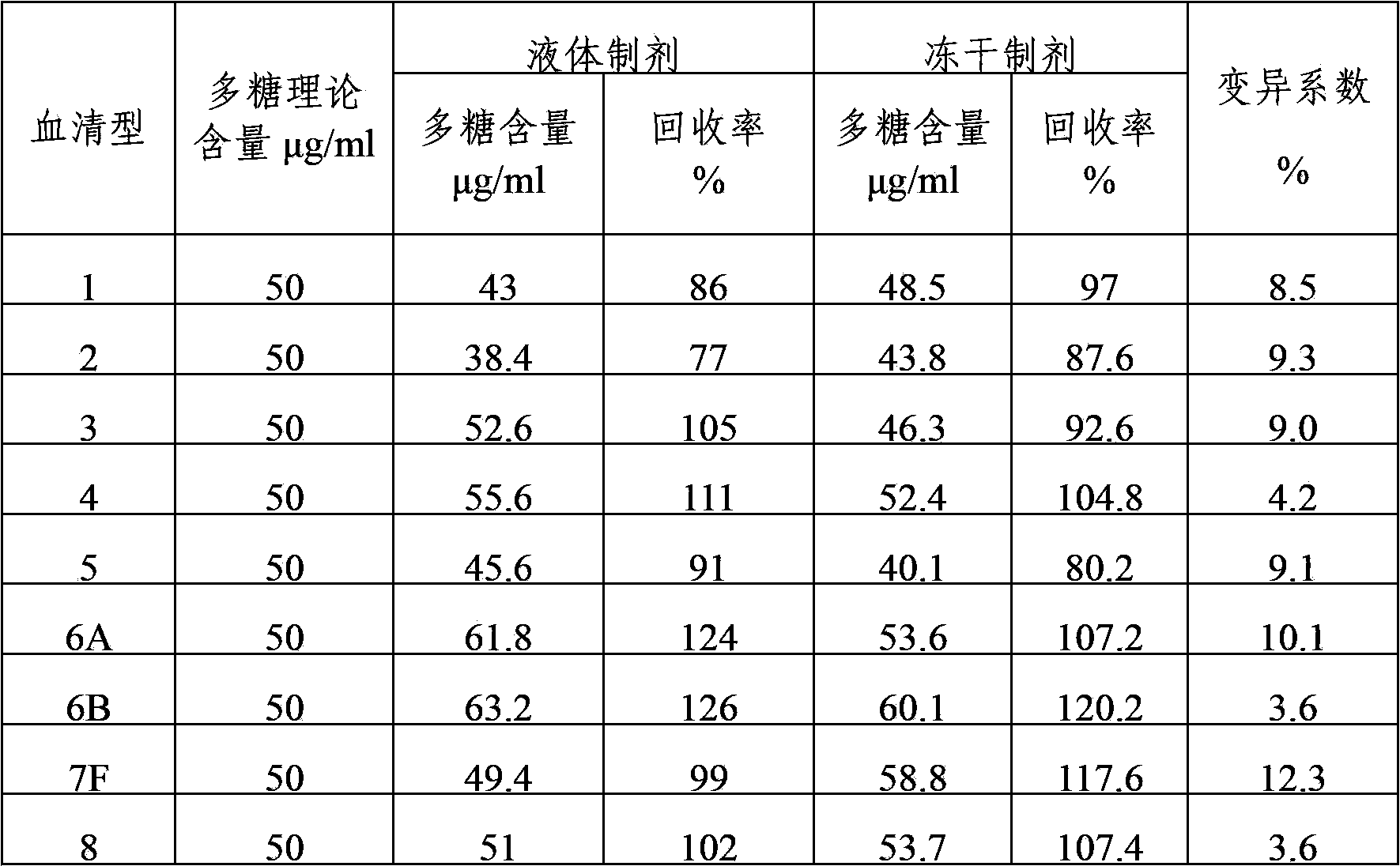
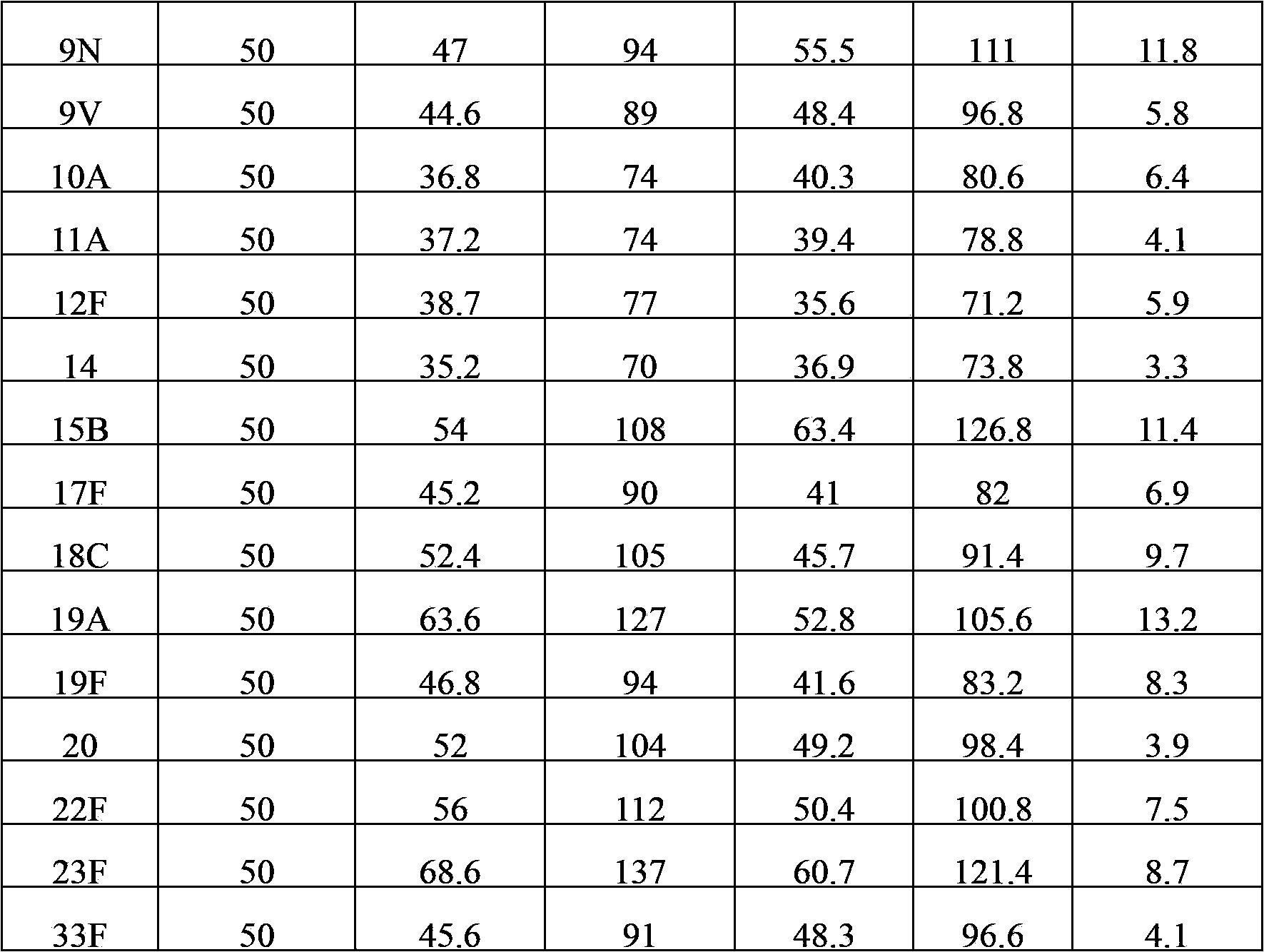
[0041] The content of various types of polysaccharides in the polyvalent pneumococcal polysaccharide composition after freeze-drying was detected by immunoturbidity method, and compared with the detection results before freeze-drying to compare the changes of antigenic su...
Embodiment 3
[0046] Example 3 Preparation of polyvalent pneumococcal polysaccharide vaccine
[0047] The multivalent pneumococcal capsular polysaccharide composition was prepared in three ways.
[0048] (1) Purified 1, 2, 3, 4, 5, 6A, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F , 23F and 33F serotype pneumococcal capsular polysaccharides were diluted to 1200 μg / ml, and then mixed in equal volumes to prepare 24-valent pneumococcal capsular polysaccharide compositions, so that the content of each type of polysaccharide was 50 μg / ml.
[0049] (2) Purified 1, 2, 3, 4, 5, 6A, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F , 23F and 33F serotype pneumococcal capsular polysaccharides are directly mixed according to the amount of various types of polysaccharides required for vaccine preparation, and then use injection water, normal saline or phosphate buffer to dilute to the vaccine preparation volume, so that the content of various types of poly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com