Tilmicosin injection as well as preparation method and application thereof
A technology of tilmicosin injection, applied in the field of tilmicosin injection and its preparation and application, can solve problems such as tachycardia and weakened contractility, reduce toxic and side effects, prevent needle infection, and simplify immunization program effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
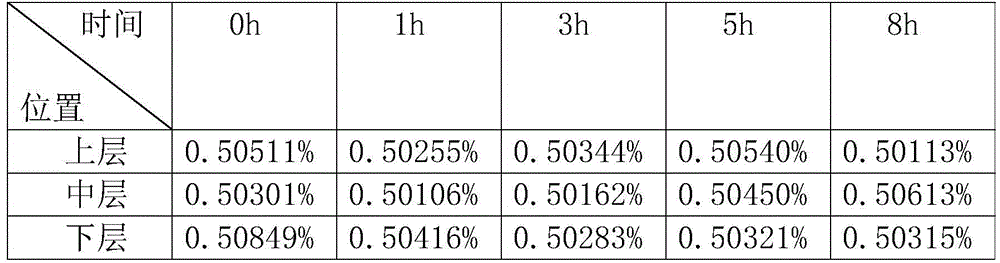
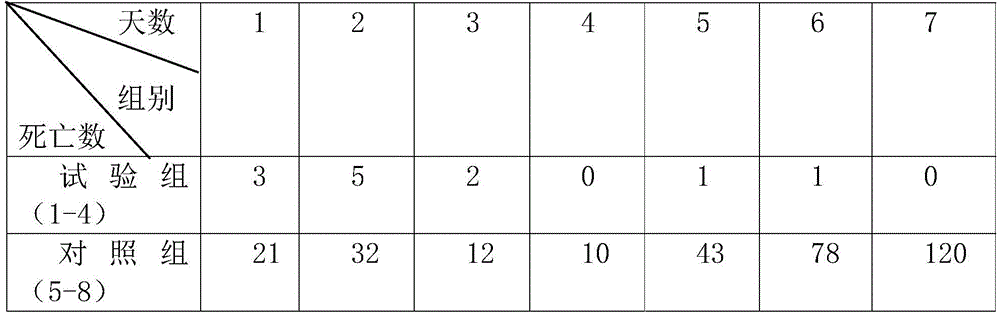
[0033] A kind of tilmicosin injection is made up of tilmicosin, astragaloside IV, ATP, cosolvent, antioxidant, stabilizer and injection water, wherein every 100ml solution contains tilmicosin 10g, astragaloside IV 1g , ATP 1g, lactic acid 5g, sodium bisulfite 0.1g, propylene glycol 15g, and water for injection to make up to 100ml.
[0034] Its preparation method is:
[0035] (1) Dissolve sodium bisulfite, ATP, and lactic acid in water for injection respectively, add tilmicosin, and stir to dissolve.
[0036] (2) Add astragaloside IV into propylene glycol and stir until dissolved. If necessary, it can be heated slowly, and the heating temperature is 55-60°C.
[0037] (3) Mix the two, adjust the pH value to 5.5 with lactic acid, dilute with water for injection, filter and sterilize.
Embodiment 2
[0039] A kind of tilmicosin injection is made up of tilmicosin, astragaloside IV, ATP, cosolvent, antioxidant, stabilizer and injection water, wherein every 100ml solution contains tilmicosin 20g, astragaloside IV 2g , ATP1.5g, citric acid 15g, Diaobaikuai 0.2g, polyethylene glycol-20015ml, water for injection to make up to 100ml.
[0040] Its preparation method is:
[0041] (1) Dissolve Diaobaikuai, ATP, and citric acid in water for injection, add tilmicosin, and stir to dissolve it.
[0042] (2) Add astragaloside IV into polyethylene glycol-200 and stir until dissolved. If necessary, it can be heated slowly, and the heating temperature is 55-60°C.
[0043] (3) Mix the two, adjust the pH value to 5.5 with citric acid, dilute with water for injection, filter and sterilize.
Embodiment 3
[0045] A kind of tilmicosin injection is made up of tilmicosin, astragaloside IV, ATP, cosolvent, antioxidant, stabilizer and injection water, wherein every 100ml solution contains tilmicosin 30g, astragaloside IV 3g , ATP 1.5g, Phosphoric Acid 12.5ml, Sodium Bisulfite 0.1g, Hangbaikuai 0.1g, Propylene Glycol 5ml, Polyethylene Glycol-200 15ml water for injection to make up to 100ml.
[0046] Its preparation method is:
[0047] Dissolve sodium bisulfite, diaobaikuai, ATP, and phosphoric acid in water for injection respectively, add tilmicosin, and stir to dissolve it.
[0048] Add astragaloside IV into the mixed solution of propylene glycol and polyethylene glycol-200 and stir until dissolved. If necessary, it can be heated slowly, and the heating temperature is 55-60°C.
[0049] Mix the two, adjust the pH value to 5.5 with phosphoric acid, dilute with water for injection, filter and sterilize.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com