Vaccine composition for preventing and treating porcine circovirus type 2, haemophilus parasuis and mycoplasma hyopneumoniae infection and preparation method thereof
A technology of mycoplasma hyopneumoniae and vaccine composition, which is applied in the direction of viral antigen components, antiviral agents, bacterial antigen components, etc., and can solve problems such as impact, increased number of immunizations, no literature and patents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
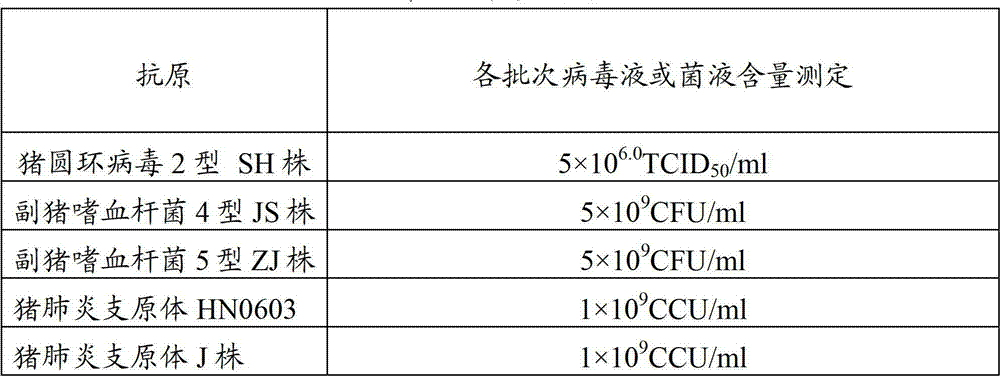
[0042] Preparation of three antigens in the vaccine composition of embodiment 1, porcine circovirus type 2, Haemophilus parasuis, and Mycoplasma hyopneumoniae
[0043] 1.1 Preparation of bacteria (virus) species for production
[0044] Preparation of porcine circovirus type 2 SH: the virus seed PCV2SH strain was diluted 1:9 with MEM liquid medium (prepared with MEM dry powder medium purchased from Invitrogen Company of the United States according to the instructions), and then divided into 5% of the volume of cell culture medium. % inoculated in PK15 (ATCC, preservation number CCL-33) monolayer cells, adsorbed at 37°C for 30 minutes, added cell maintenance solution (4% calf serum and 2mmol / L D-glucosamine hydrochloride were added to MEM liquid medium) ), cultivated at 37°C for 4 days, freeze-thawed 2-3 times, harvested the virus, and the virus titer was 10 6.5 TCID 50 / ml.
[0045] Preparation of Haemophilus parasuis strains: Streak inoculation of Haemophilus parasuis type ...
Embodiment 2
[0092] Embodiment 2, the preparation of porcine circovirus antigen, Haemophilus parasuis antigen, mycoplasma swine pneumonia antigen vaccine composition:
[0093] In order to compare the immune effects of vaccine compositions with different antigen contents, 4 kinds of adjuvants were selected to prepare the vaccine compositions, namely Montanide TM Gel (produced by SEPPIC, France), aluminum glue, white oil, squalene, each adjuvant was used to prepare porcine circovirus antigen, Haemophilus parasuis antigen, mycoplasma pneumoniae antigen vaccine composition and porcine round Circovirus disease, Haemophilus parasuis disease, and mycoplasma pneumoniae single vaccine, in which two vaccines with gradient antigen content were prepared for each single vaccine, and the antigen content of a single vaccine was the same as that of porcine circovirus disease, Haemophilus parasuis disease, mycoplasma pneumonia vaccine composition of the present invention (or claim the vaccine composition ...
Embodiment 3
[0133] Embodiment 3, porcine circovirus type 2 antigen, Haemophilus parasuis antigen, Mycoplasma hyopneumoniae antigen vaccine composition and the vaccine composition vaccine of each individual antigen The comparative test of immune effect on pigs
[0134] 3.1 Piglets immunized with vaccines for the test
[0135] Vaccine compositions (vaccine 1, vaccine 1', vaccine 8, vaccine 15, vaccine 22) of different adjuvants porcine circovirus type 2, Haemophilus parasuis, and mycoplasma hyopneumoniae prepared by the method described in Example 2 and Porcine circovirus type 2 inactivated vaccines (vaccine 2, vaccine 3, vaccine 9, vaccine 10, vaccine 16, vaccine 17, vaccine 23, vaccine 24), inactivated vaccines for porcine haemophilus parasuis (vaccine 4, vaccine 5. Vaccine 11, Vaccine 12, Vaccine 18, Vaccine 19, Vaccine 25, Vaccine 26), Inactivated Vaccine of Swine Mycoplasma Pneumonia (Vaccine 6, Vaccine 6', Vaccine 7, Vaccine 7', Vaccine 13, Vaccine 14, Vaccine 20, Vaccine 21, Vaccine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com