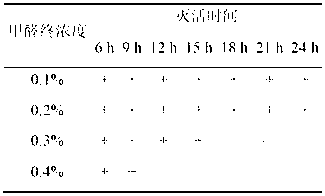
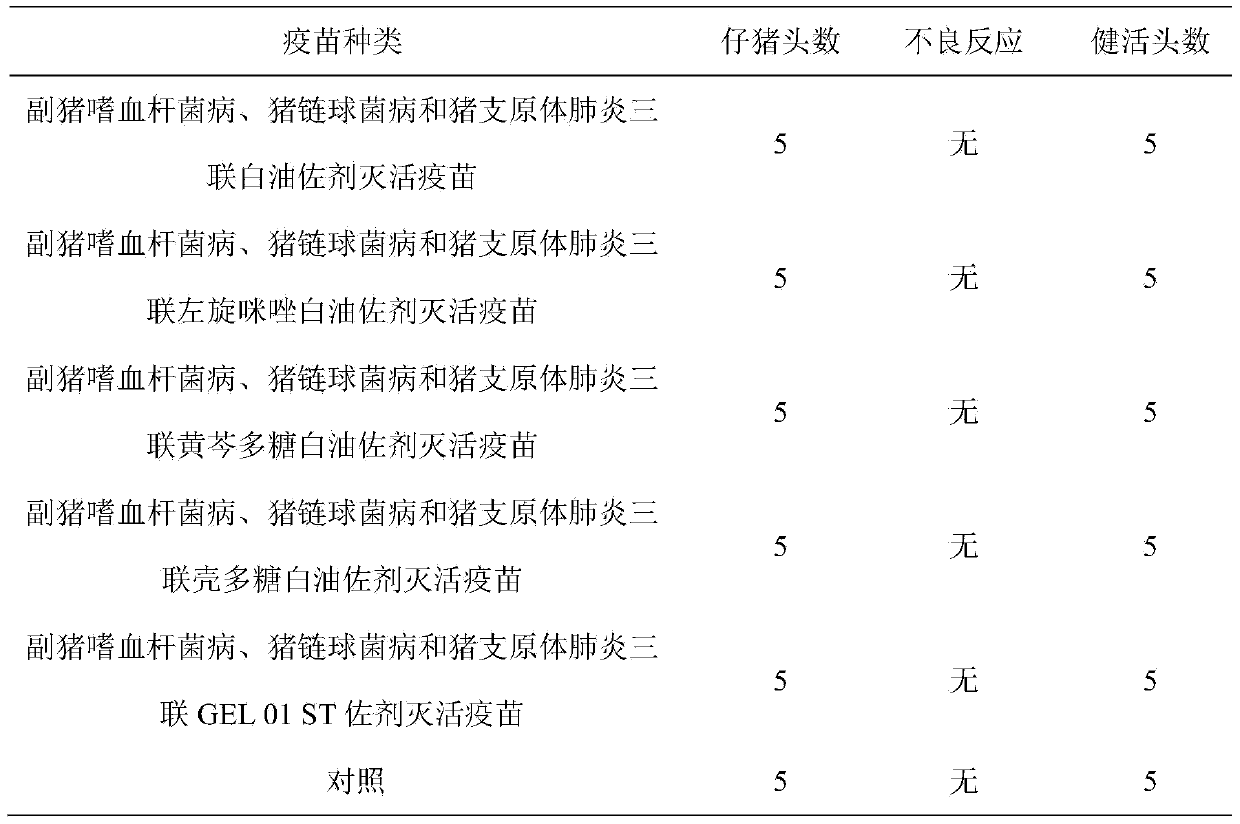
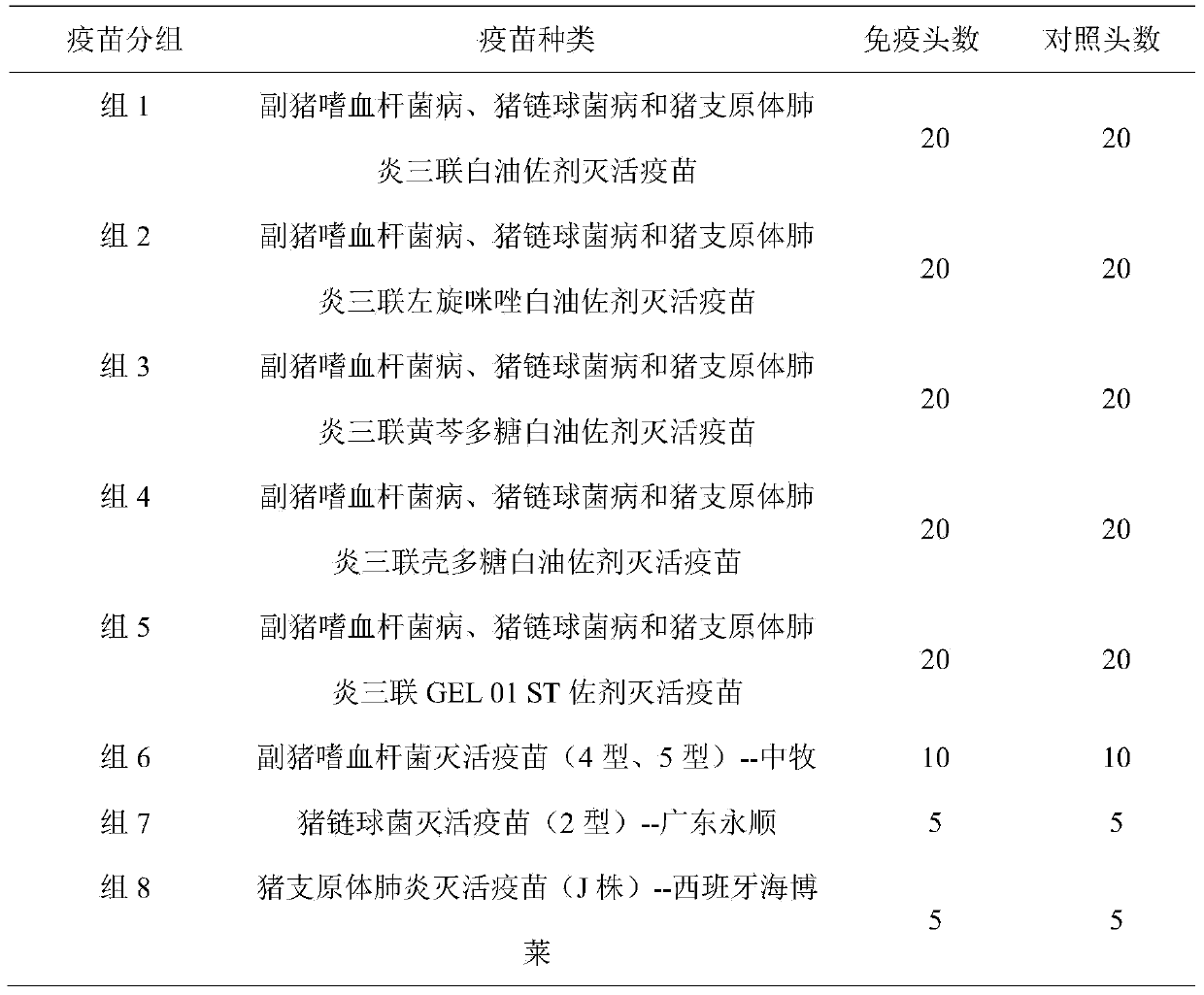
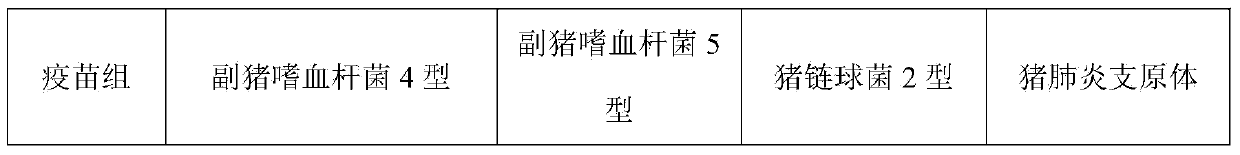
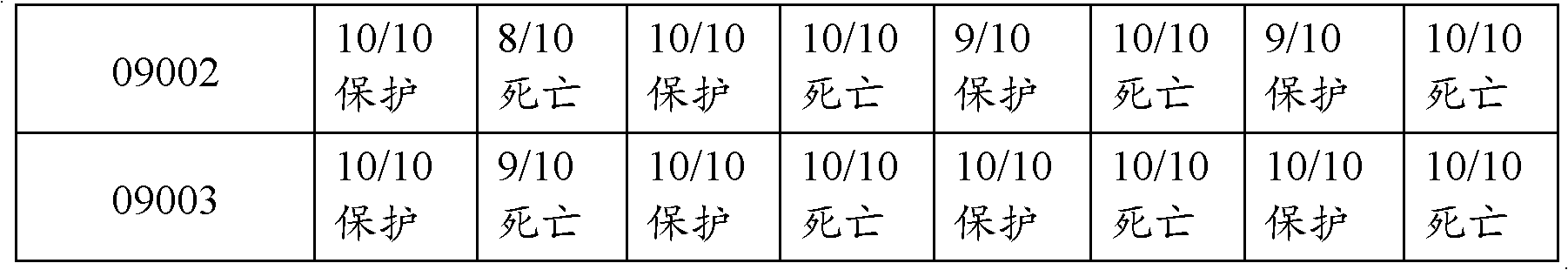
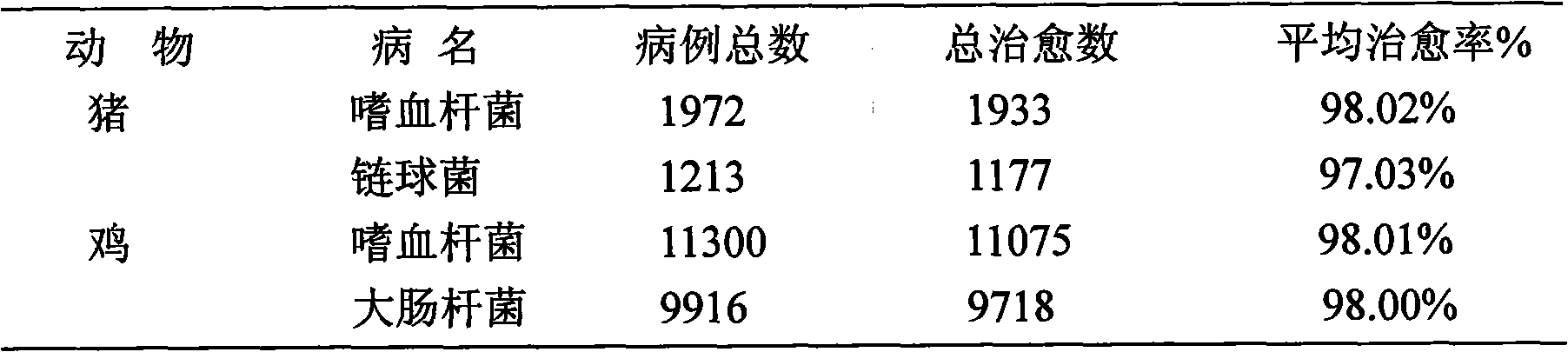
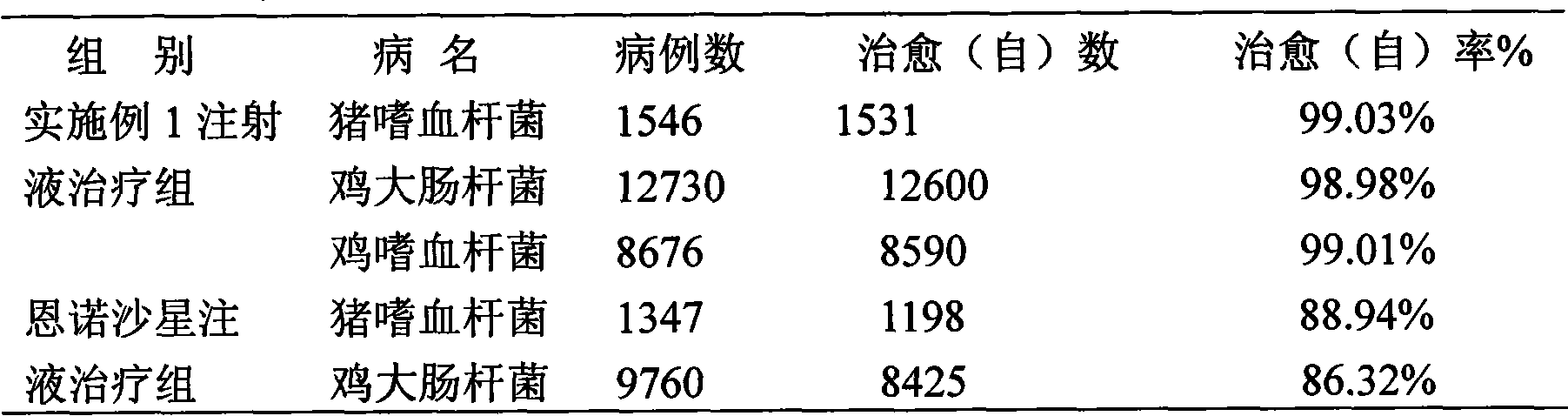
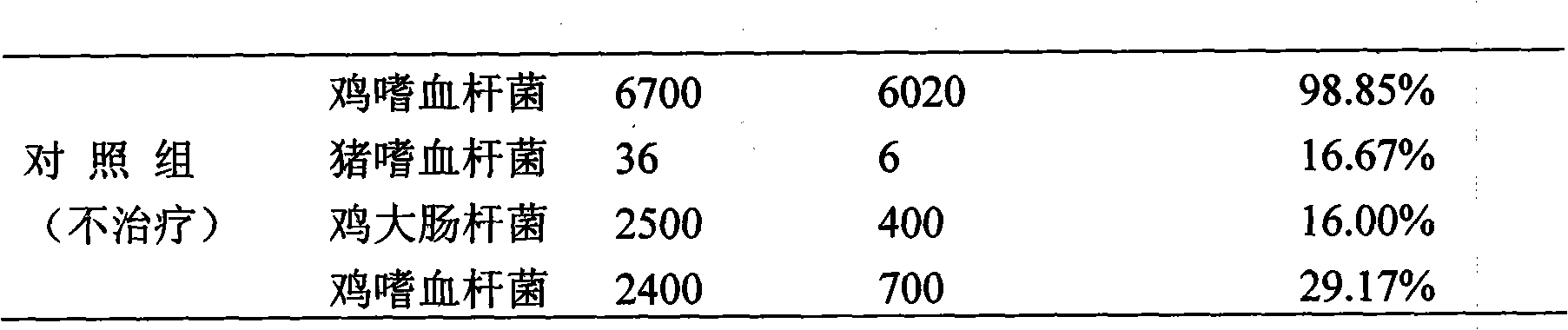
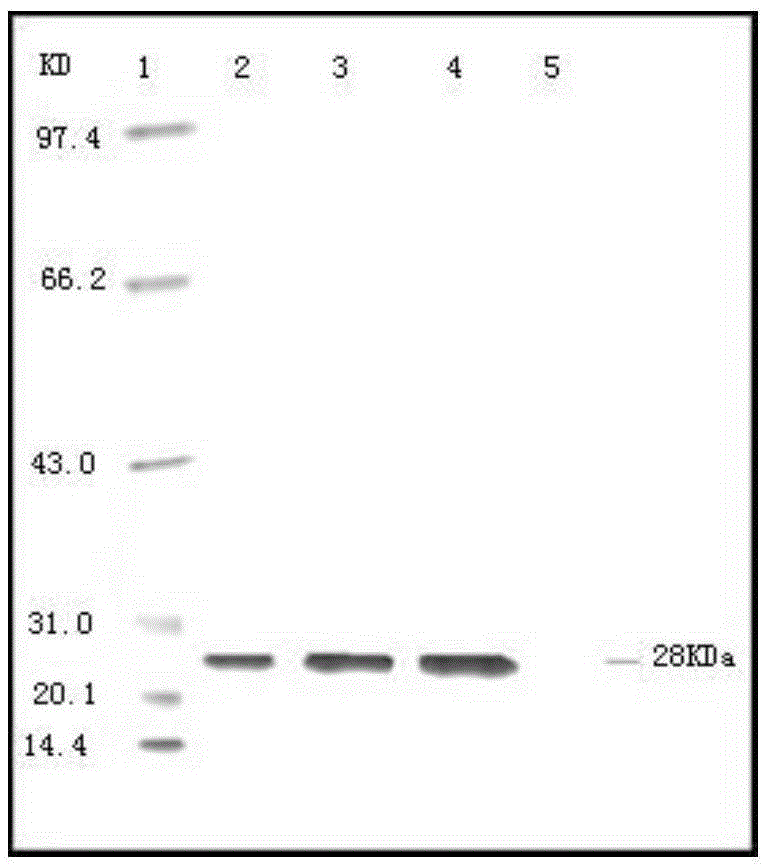
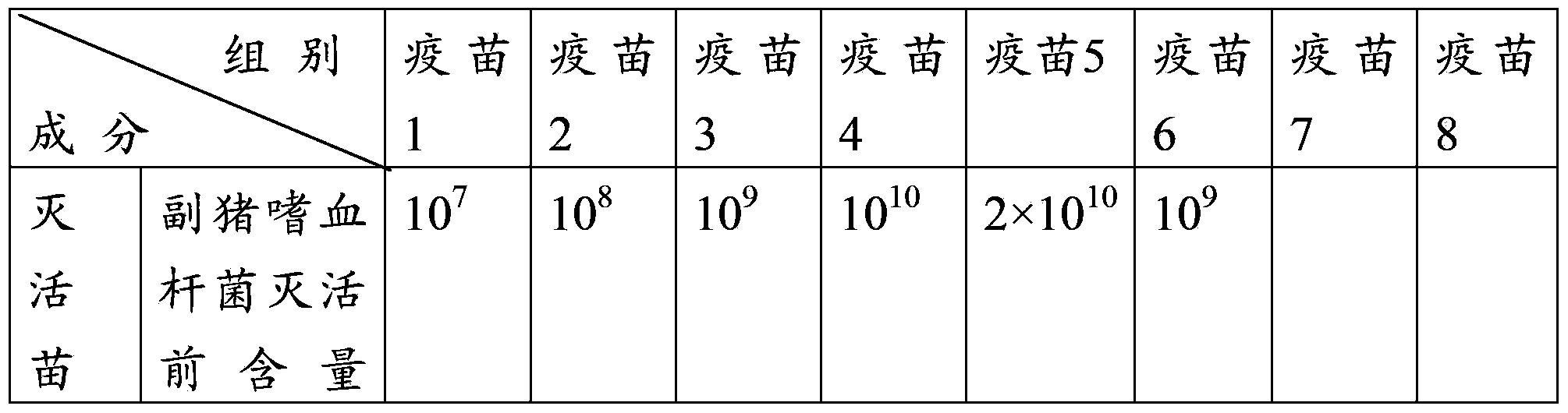
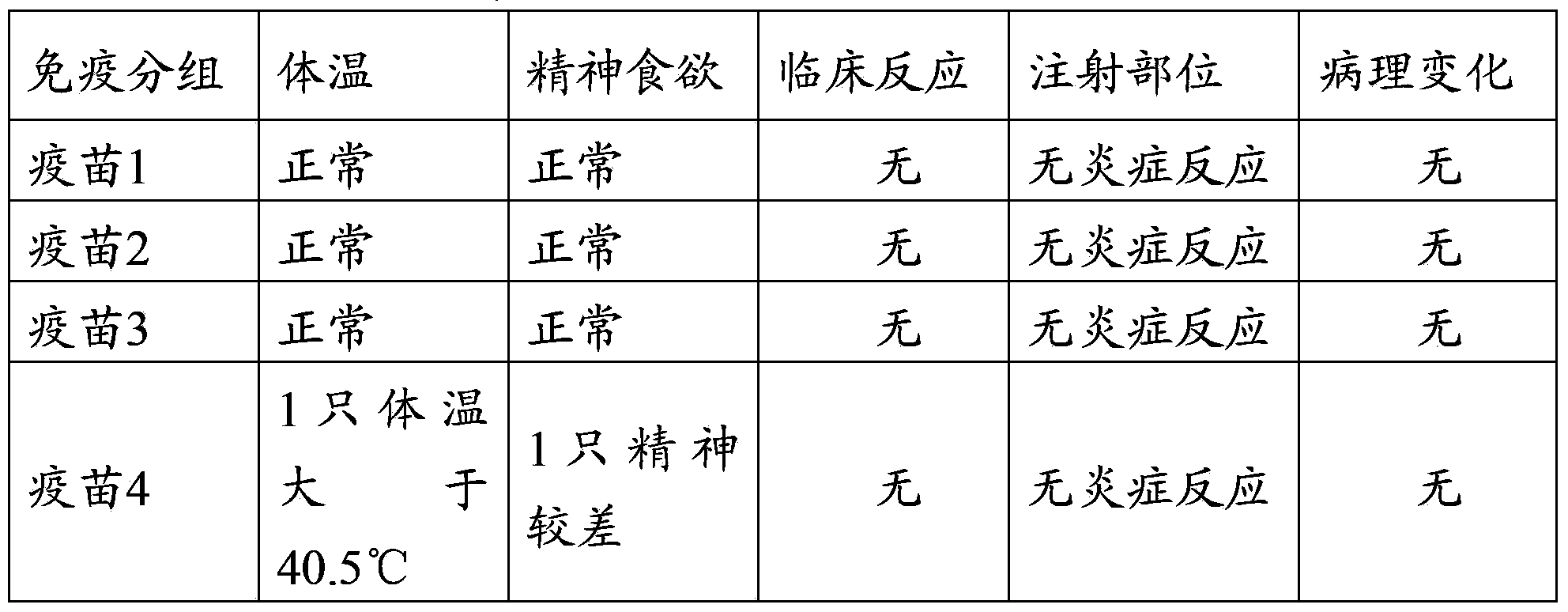
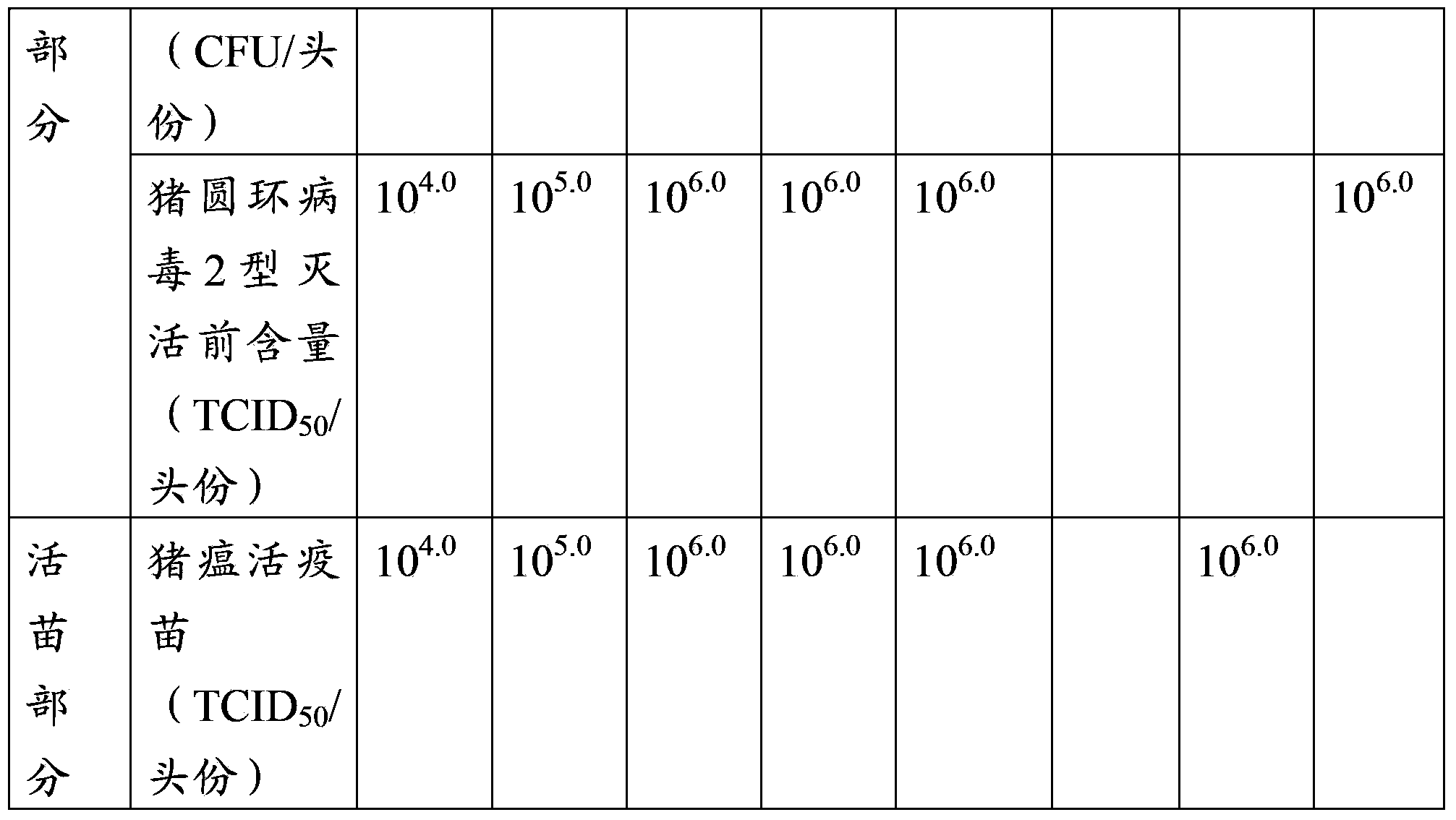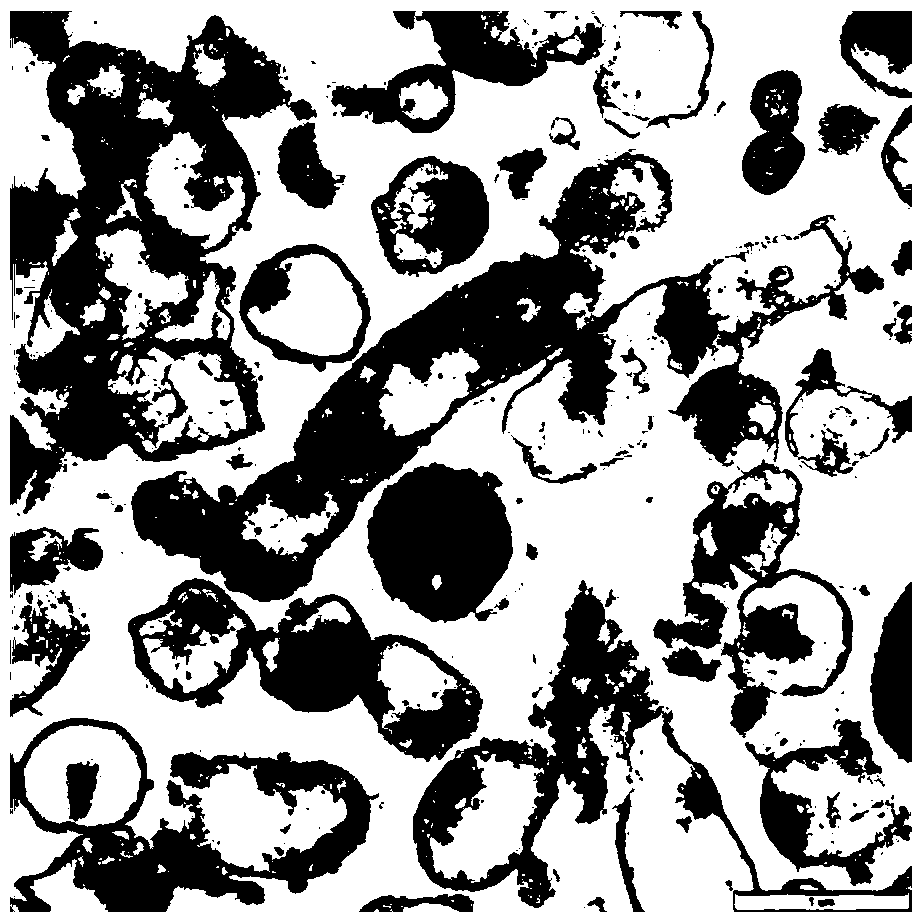Patents
Literature
72 results about "Haemerosia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Haemerosia is a genus of moths of the family Noctuidae.
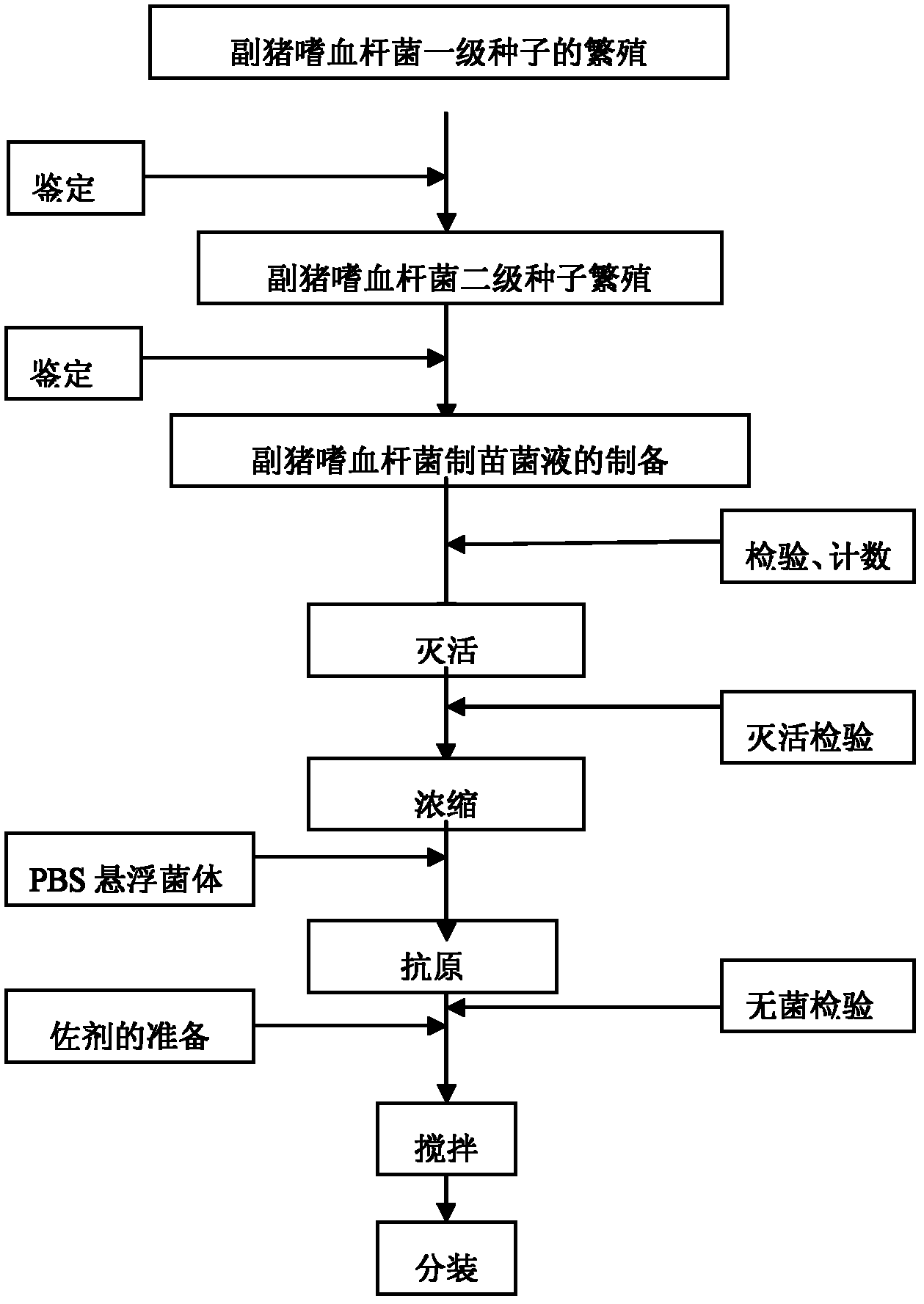
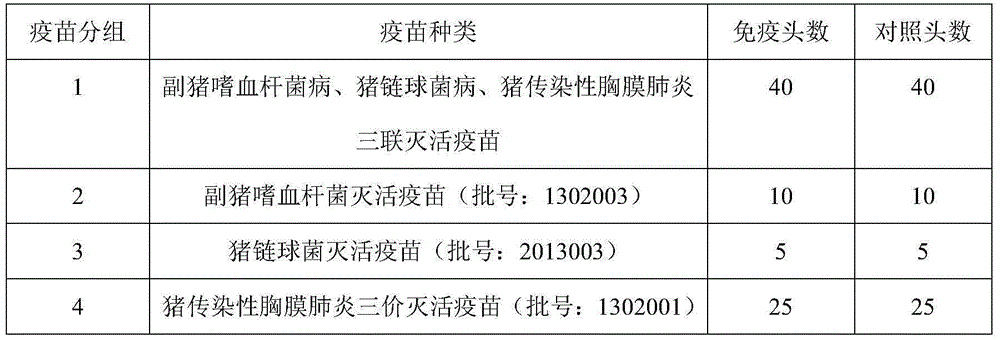
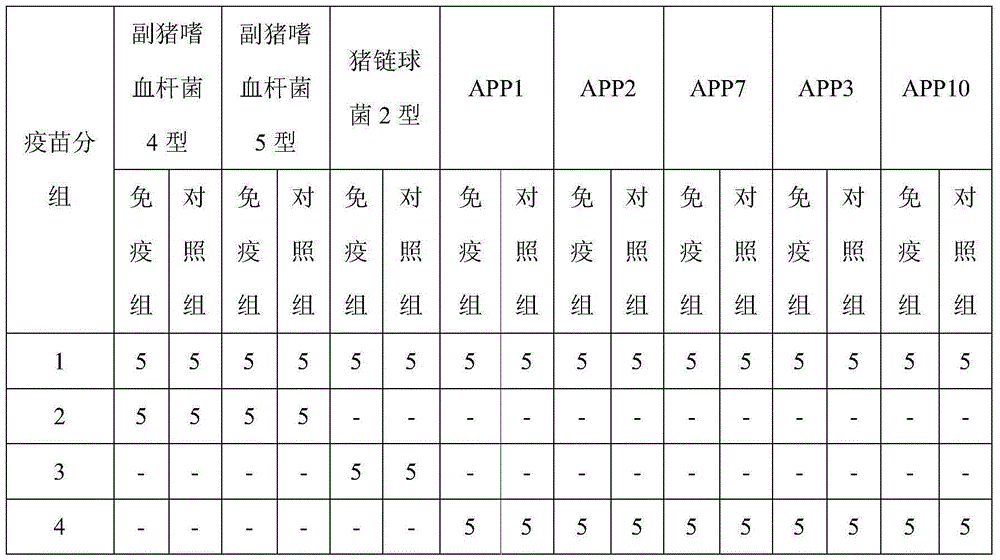
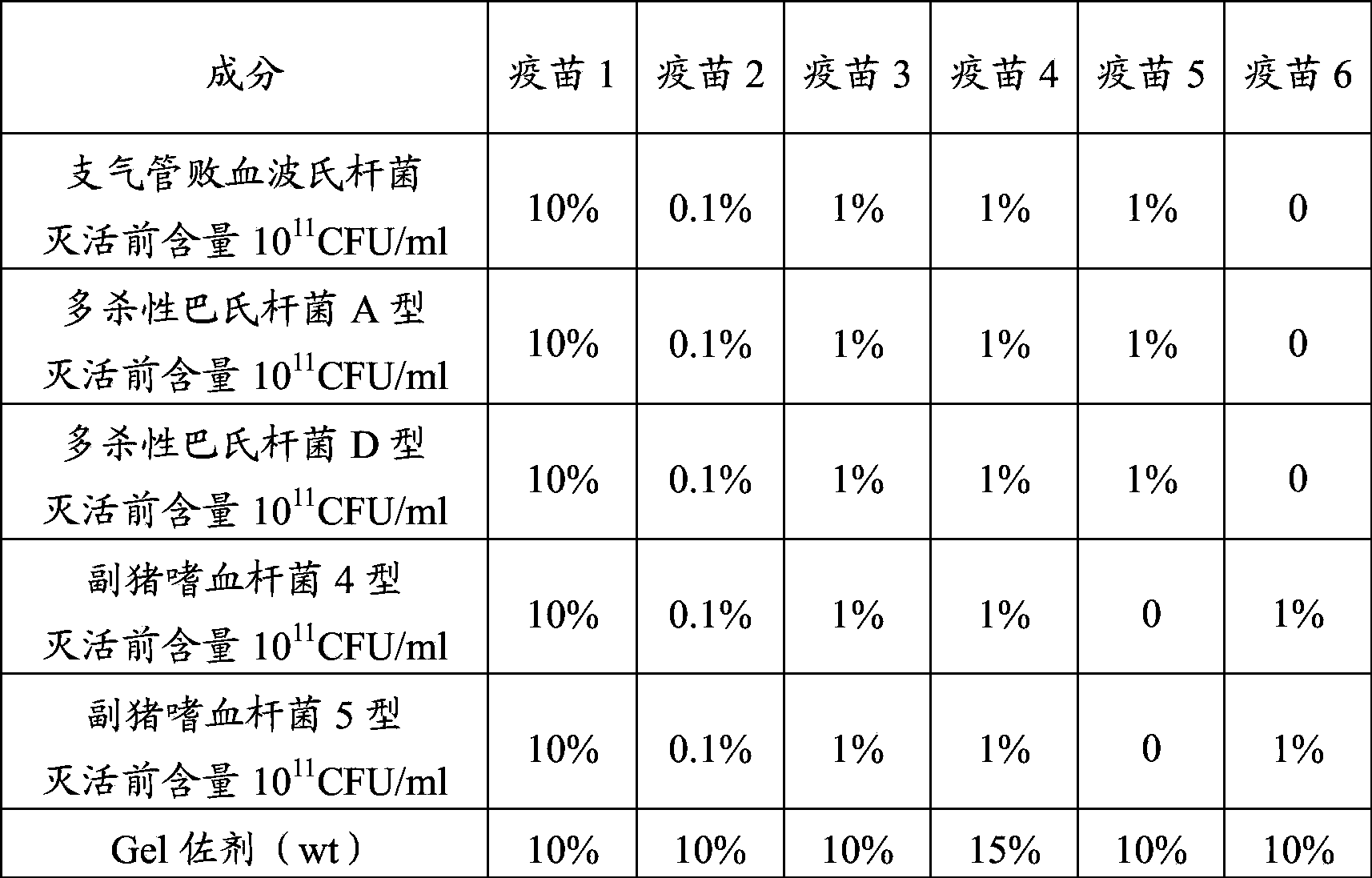
Novel haemophilus parasuis disease trivalent inactivated vaccine and preparation method thereof
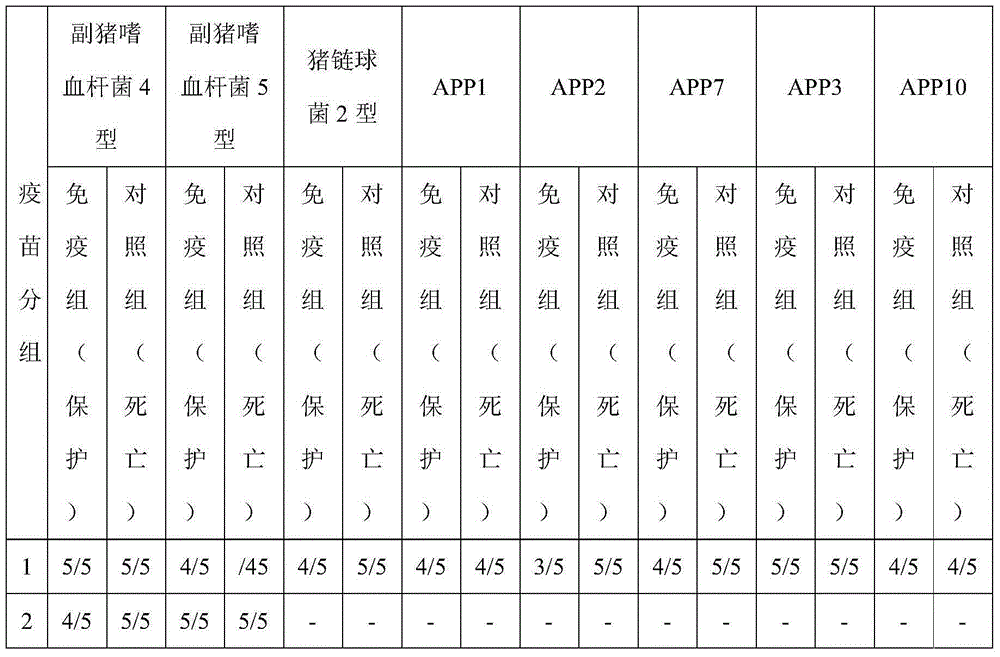
ActiveCN102908615ASolve preparation difficultiesImprove immunityAntibacterial agentsBacterial antigen ingredientsAntigenDisease
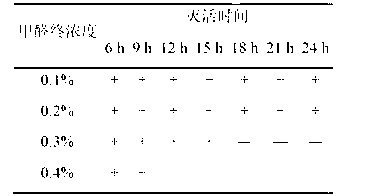
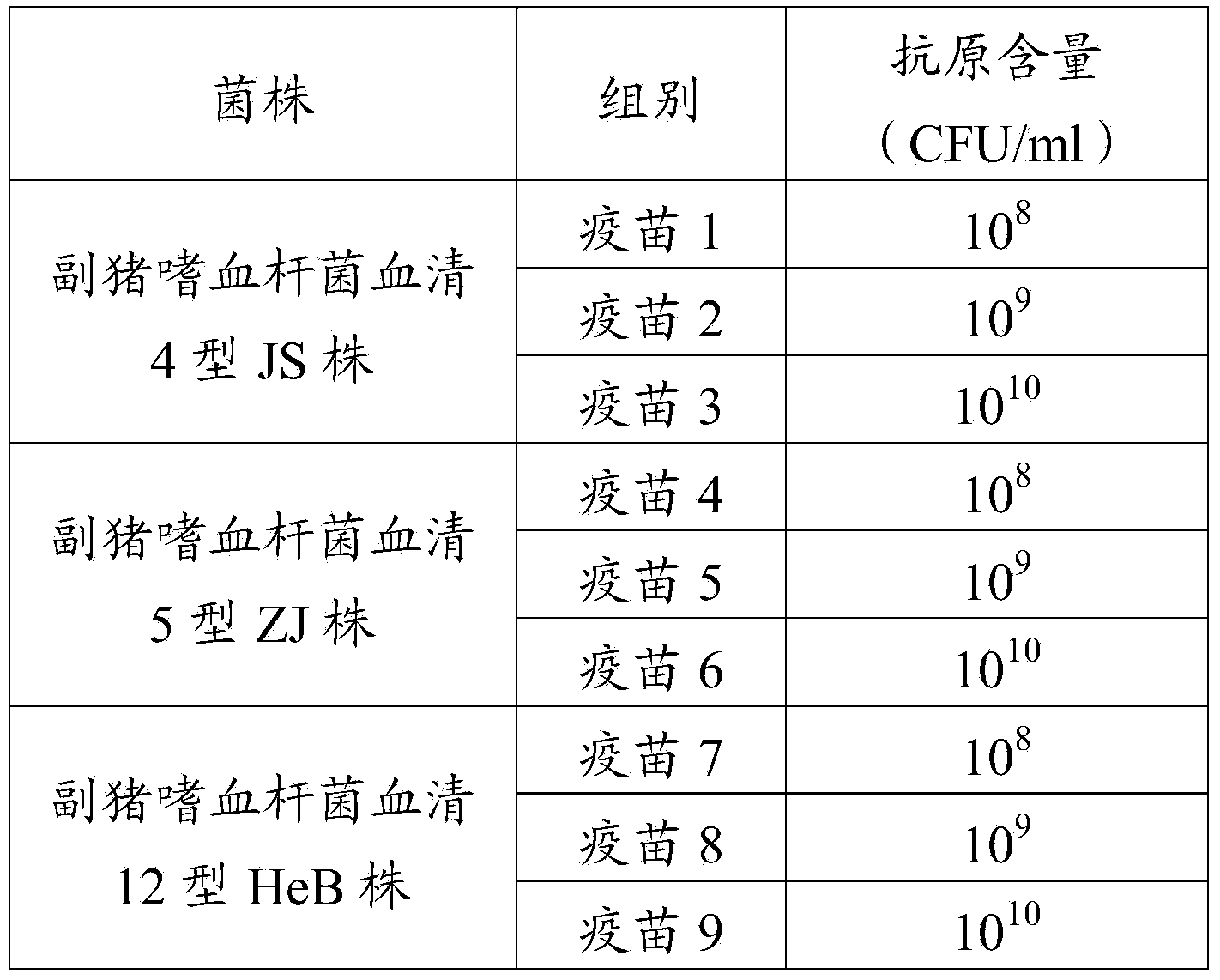
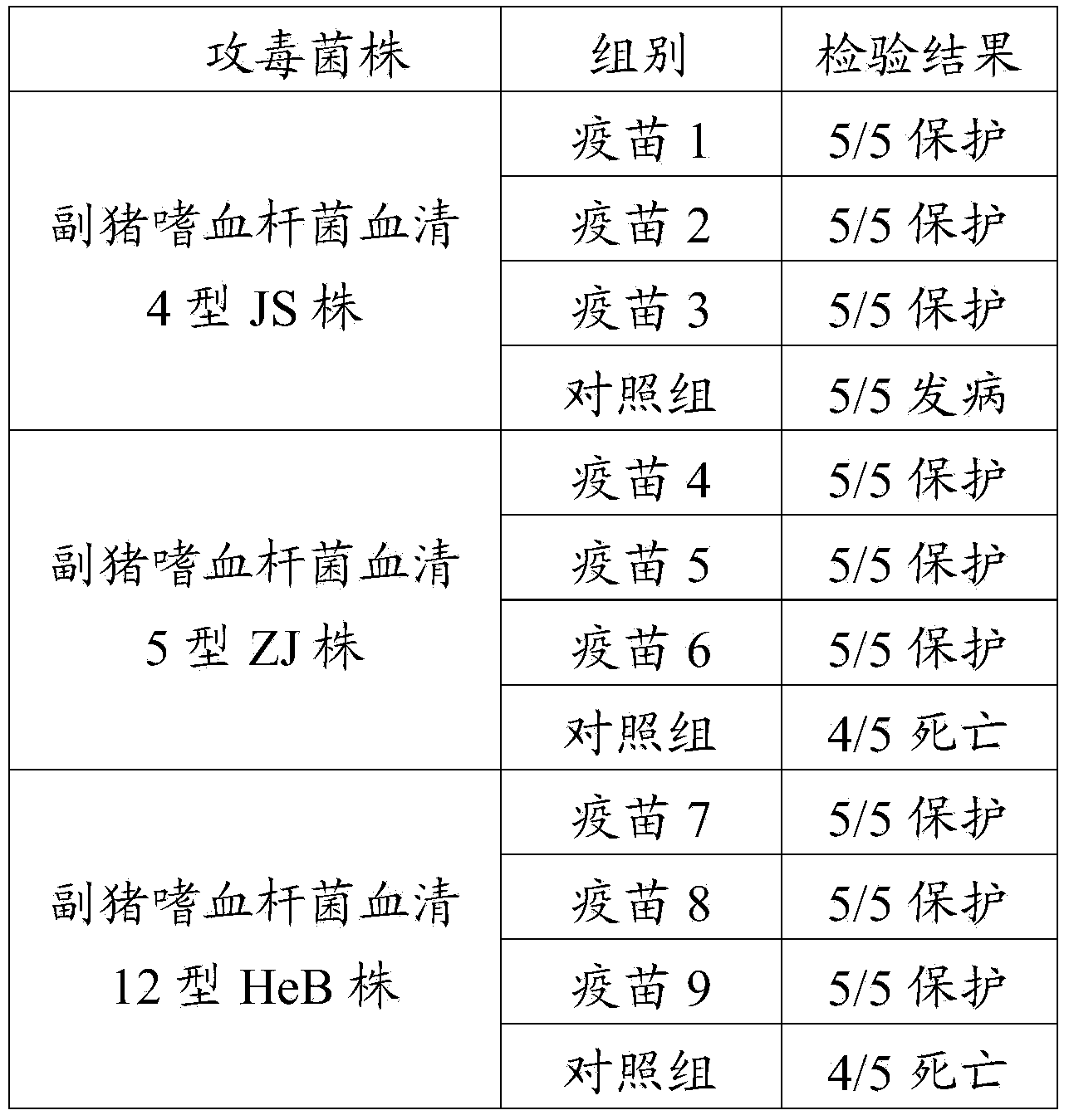
The invention provides a novel haemophilus parasuis disease trivalent inactivated vaccine and a preparation method thereof. The vaccine comprises equal proportions of antigens of: inactivated haemophilus parasuis serotype 4 JS strain, inactivated haemophilus parasuis serotype 5 ZJ strain, and inactivated haemophilus parasuis serotype 12 HeB strain. According to the invention, the haemophilus parasuis serotype 4, serotype 5, and serotype 12 strains are obtained by separation. Concentration contents and relative proportions of the antigens prepared from the three strains are subjected to large amounts of woks and practices, such that the trivalent vaccine with an appropriate antigen ratio and an appropriate concentration is obtained. The trivalent vaccine has good preventing and treating effects against haemophilus parasuis diseases caused by various epidemic haemophilus parasuis serotypes in our nation. Especially, the trivalent vaccine can solve a problem of poor treatment effect of existing vaccines caused by novel haemophilus parasuis pathogens.
Owner:PU LIKE BIO ENG
Vaccine composition for preventing and treating porcine circovirus type 2, haemophilus parasuis and mycoplasma hyopneumoniae infection and preparation method thereof
ActiveCN103083655ASimplified immunization programReduce manufacturing costAntibacterial agentsBacterial antigen ingredientsDiseaseCircovirus
The invention provides a vaccine composition for preventing and treating porcine circovirus type 2, haemophilus parasuis and mycoplasma hyopneumoniae infection. The vaccine composition comprises an inactivated porcine circovirus type 2 antigen, inactivated haemophilus parasuis, inactivated mycoplasma hyopneumoniae and a vaccine adjuvant. The vaccine composition disclosed by the invention can realize the aim of preventing three diseases including a porcine circovirus disease, mycoplasma pneumonia, a haemophilus parasuis disease by one injection of the vaccine; the content of antigen is 1 / 2 of the content of a common single-vaccine antigen when the vaccine composition disclosed by the invention is prepared by mixing the three antigens; and compared with the existing condition that three injections of single vaccine are injected to prevent three infectious diseases, the technical scheme disclosed by the invention is economical and practical, reduces the production cost, simplifies an immune procedure and reduces the epidemic prevention cost.
Owner:PU LIKE BIO ENG
Porcine streptococcus disease and haemophilus parasuis disease combined inactivate vaccine and preparation method thereof
ActiveCN102329746AReduce stressEasy to useAntibacterial agentsBacterial antigen ingredientsHaemophilusAluminium stearate
The invention discloses a porcine streptococcus disease and haemophilus parasuis disease combined inactivate vaccine and a preparation method thereof. The preparation method comprises the following steps of: a, respectively carrying out enrichment culture on a porcine streptococcus strain, a haemophilus parasuis strain and a haemophilus parasuis strain to obtain a porcine streptococcus strain bacterial solution, a haemophilus parasuis strain bacterial solution and a haemophilus parasuis strain bacterial solution; b, respectively adding a formaldehyde solution into the porcine streptococcus strain bacterial solution, the haemophilus parasuis strain bacterial solution and the haemophilus parasuis strain bacterial solution, and inactivating; c, mixing the collected porcine streptococcus strain bacterial solution, the haemophilus parasuis strain bacterial solution and the haemophilus parasuis strain bacterial solution, adding Tween-80 for preparing a water phase, preparing white oil, Span-80 and aluminium stearate into an oil phase, mixing the water phase with the oil phase to prepare a uniform emulsion, i.e. an oil emulsion inactivating vaccine; and 4, sub-packaging the oil emulsion inactivating vaccine. The porcine streptococcus disease and haemophilus parasuis disease combined inactivate vaccine can effectively prevent the porcine streptococcus disease and haemophilus parasuis disease, does not have hidden danger of scattering viruses and is safe and reliable; and the immunization is realized by one vaccine, thus the cost is reduced.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Vaccine composition containing porcine circovirus type 2 antigen and haemophilus parasuis antigen, as well as preparation method and application thereof
ActiveCN102988978AChange cognitive biasImprove immunityAntibacterial agentsBacteriaAntigenHaemophilus
The invention relates to a polyvalent vaccine composition for porcine, and in particular relates to a vaccine composition capable of resisting infection of porcine circovirus type 2 (PCV 2) and haemophilus parasuis (HPS) at the same time. The vaccine composition comprises at least one PCV2 antigen, at least one HPS, as well as vector, an excipient and an adjuvant available in the field of veterinary medicine. The vaccine composition can be used for preventing and treating PCV2 related diseases and HPS diseases.
Owner:PU LIKE BIO ENG
Hemophilus parasuis disease, swine streptococcosis bivalent inactivated vaccine and preparation method thereof
ActiveCN103157100ALittle side effectsFulfil requirementsAntibacterial agentsBacterial antigen ingredientsDiseaseAntigen
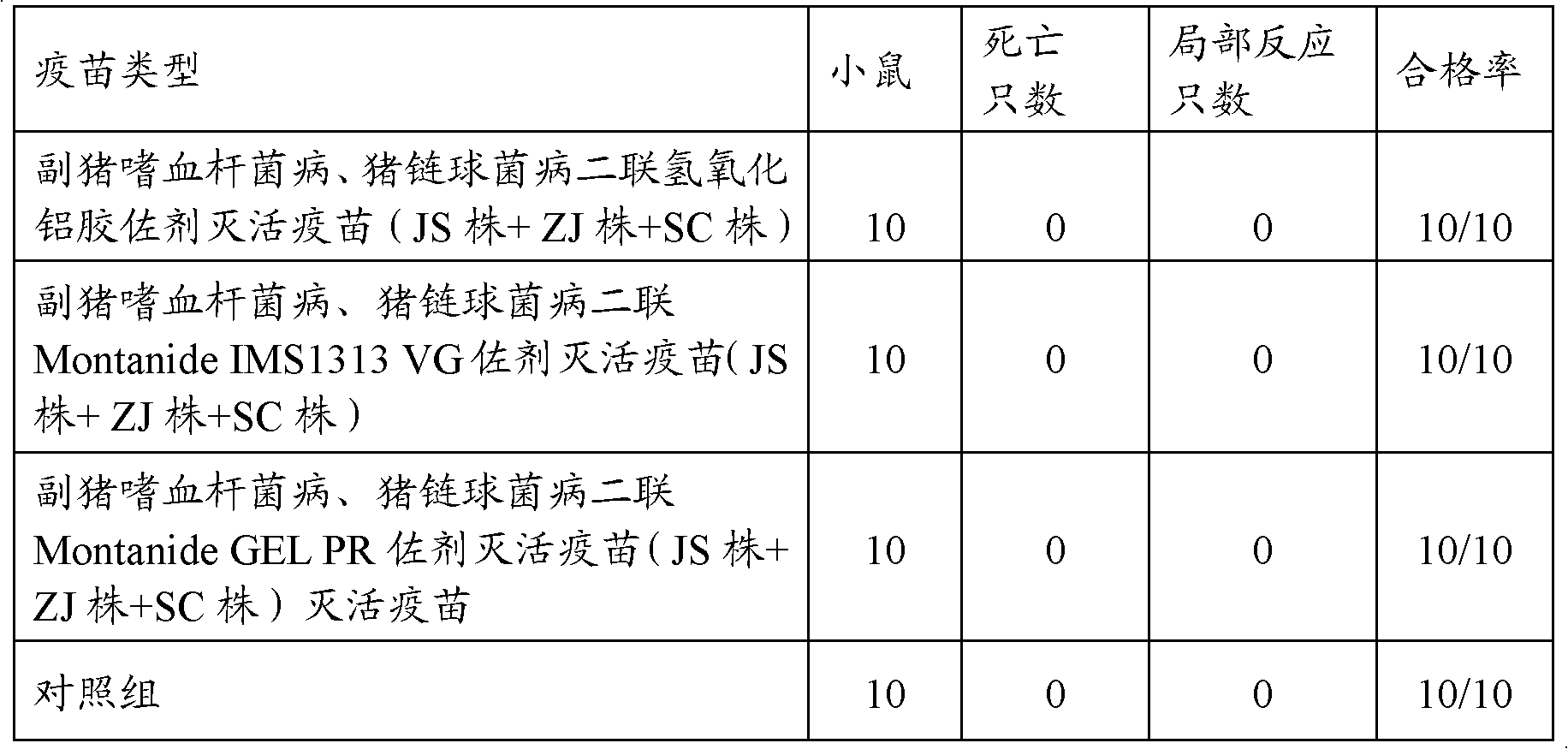
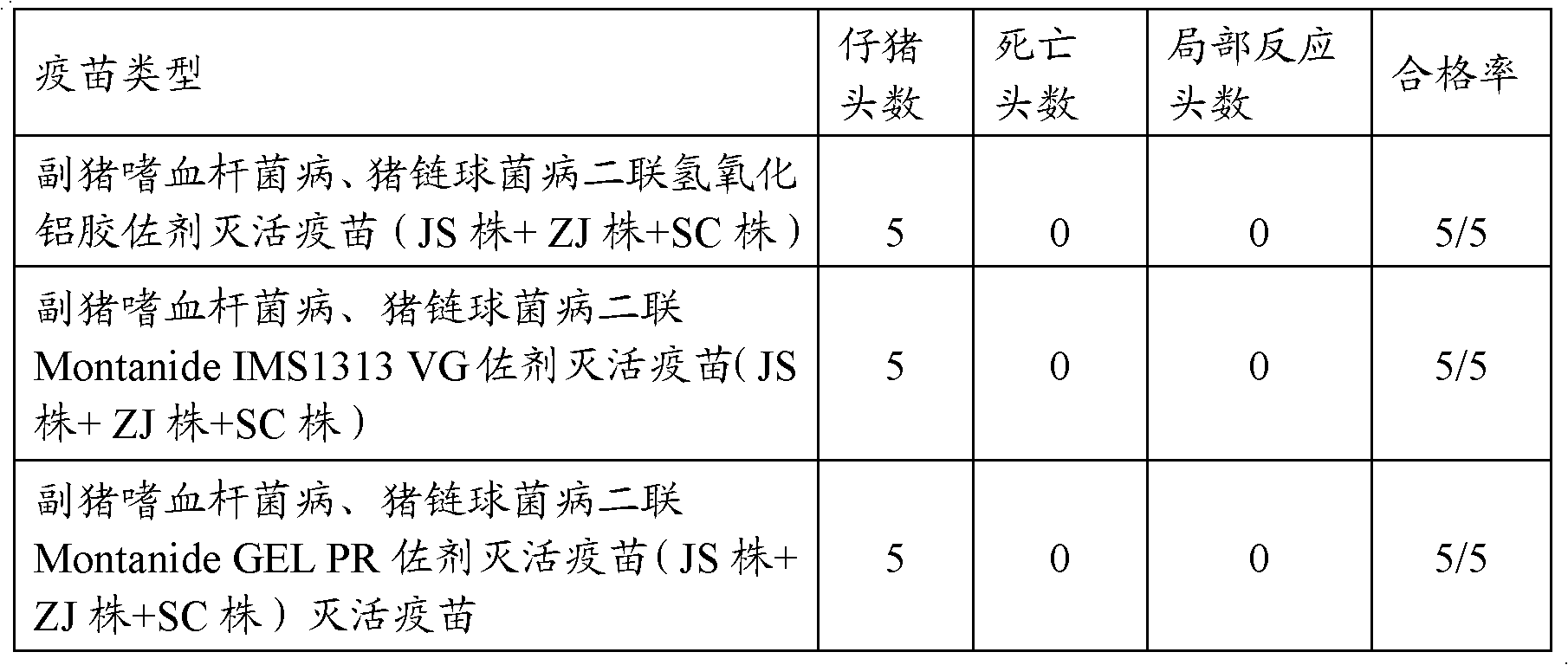
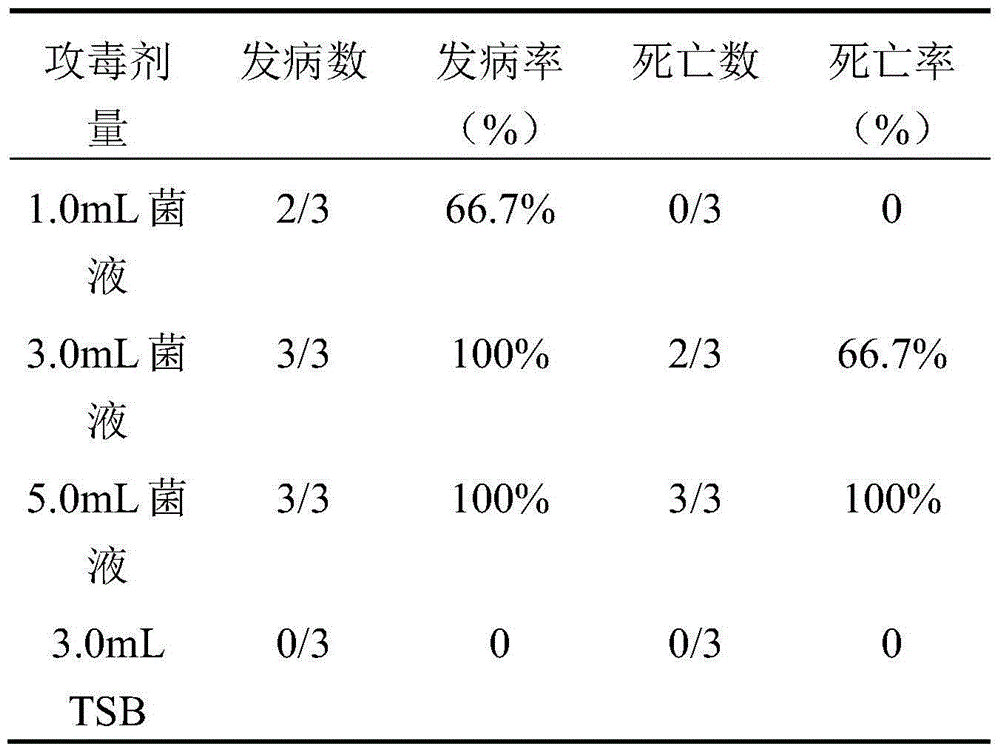
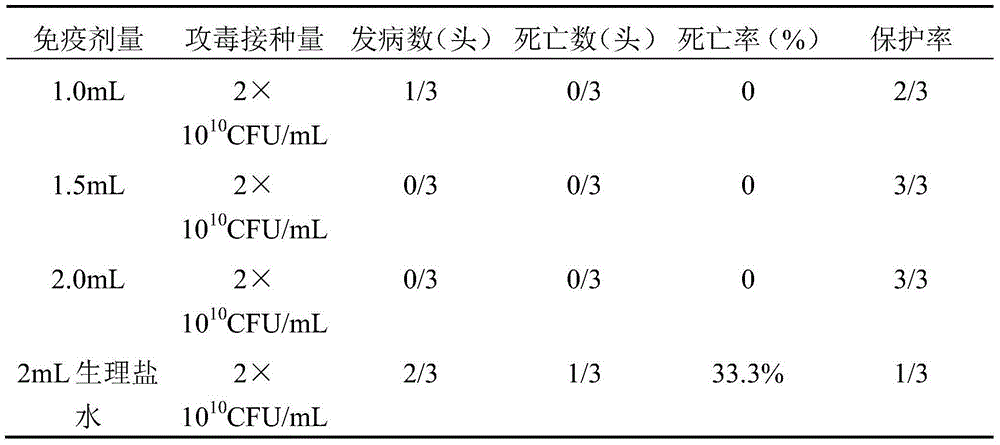
The present invention relates to a hemophilus parasuis disease, a swine streptococcosis bivalent inactivated vaccine and a preparation method thereof. The vaccine is prepared by adopting a hemophilus parasuis serotype 4 JS strain, a hemophilus parasuis serotype 5 ZJ strain and a streptococcus suis type 2 SC strain as antigens, and adopting a veterinarily acceptable adjuvant, concurrently provides immunoprophylaxis for hemophilus parasuis diseases caused by hemophilus parasuis, swine streptococcosis caused by streptococcus suis, and mixed infection of the hemophilus parasuis disease and the swine streptococcosis, has effects of low side effect, no endotoxin, no impurity protein in serum, good immunization safety, multi-prevention effect with one needle in the clinic, and cost reducing, and can meet different requirements of different users.
Owner:PU LIKE BIO ENG
Haemophilus parasuis and application thereof
InactiveCN104611274AImprove protectionStable biological propertiesAntibacterial agentsBacteriaDiseaseImmune effects
The invention aims to provide haemophilus parasuis and application thereof. The preservation number of haemophilus parasuis is CGMCC No. 10230. The haemophilus parasuis is used for preparing medicines for treating haemophilus parasuis. A haemophilus parasuis serum type 5 vaccine strain LX-5 has a stable biological characteristic, has relatively strong pathogenicity to a piglet, and has good immunogenicity. A propolis inactivated vaccine prepared by applying the haemophilus parasuis is safe and reliable and can generate an antibody in a relatively high level, is long in duration and has a good protective effect on the piglet counteracting toxic substances for homologous strains. The morbidity and the death rate of the immunized swinery are obviously reduced, and the immune effect of the vaccine reaches or is superior to existing commercialized vaccines in the market, so that prevalence and spread of the haemophilus parasuis can be effectively prevented, the economical loss caused by the disease is lowered, and the haemophilus parasuis has a broad application prospect.
Owner:QINGDAO AGRI UNIV
Trigeminy inactivated vaccine for porcine circovirus disease, porcine streptococcus suis disease and porcine haemophilus parasuis disease, preparation method of the vaccine and applications of the vaccine
InactiveCN103409374APromote mass replicationImprove reproductive performanceAntibacterial agentsAntiviralsHaemophilusCircovirus
The invention discloses a trigeminy inactivated vaccine for porcine circovirus disease, porcine streptococcus suis disease and porcine haemophilus parasuis disease, a preparation method of the vaccine and applications of the vaccine. Porcine circovirus 2-WH has an accession number of CCTCC NO: V20133 and has strong virulence, good immunogenicity, a high antigen titer, and a high virulence reaching 10<7.4>TCID[50] / mL. The trigeminy inactivated vaccine, which is prepared by mixing the porcine circovirus 2-WH, porcine streptococcus suis 2-LT, and porcine haemophilus parasuis 4-MD0322 and porcine haemophilus parasuis 5-SH0165, can simultaneously prevent the porcine circovirus disease and diseases caused by porcine streptococcus suis 2-type, porcine haemophilus parasuis 4-type and porcine haemophilus parasuis 5-type, the immune protective effect on the same serotype reaches over 80%.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Serotype 5 haemophilus parasuis (HPs) vaccine strain
ActiveCN103194412AStable biological propertiesEpidemic preventionAntibacterial agentsBacteriaMortality rateHaemophilus Vaccines
The invention relates to the field of vaccines of Haemophilus parasuis (HPs) in veterinary biological products and discloses a serotype 5 HPs vaccine strain. The class name of the vaccine strain is HPs. The strain number is XX0306. The vaccine strain has been collected in the China Center for Type Culture Collection, with collection number being CCTCC NO:M2013095 and collection date being March 21, 2013. The serotype 5 HPs strain XX0306 has stronger pathogenicity toward swine and has good immunogenicity. The inactivated vaccines prepared from the vaccine strain are safe and reliable and have quite good protective effects on the swine challenging homological strains. The morbidity and mortality of the immunized swinery are obviously reduced. Either the single vaccine or the combined vaccine has the immune effects equivalent or superior to the immune effects of the existing commercialized vaccines and can effectively prevent prevalence of HPs.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Method for preparing triple inactivated vaccine
InactiveCN104208667AReduce stressLow costAntibacterial agentsBacterial antigen ingredientsDiseaseHaemophilus
The invention provides a method for preparing a triple inactivated vaccine. The triple inactivated vaccine has a relatively good immunizing effect on haemophilus parasuis, swine streptococcosis and mycoplasma pneumoniae of swine, and the aims of preventing multiple diseases by one injection, reducing cost and reducing swine stress.
Owner:TIANJIN RINGPU BIO TECH
Haemophilus parasuis disease antibody detecting test strip and preparation method thereof
The invention discloses a haemophilus parasuis disease antibody detecting test strip and a preparation method thereof. The haemophilus parasuis disease antibody detecting test strip consists of a PVC (polyvinyl chloride) lining plate, a nitrocellulose membrane, an absorbing pad, a gold label pad and a sample pad, wherein the PVC lining plate is arranged at the very bottom; the nitrocellulose membrane is arranged on the middle section of the upper part of the PVC lining plate; the absorbing pad is arranged at the left end of the upper part of the nitrocellulose membrane; the gold label pad is arranged at the right end of the upper part of the nitrocellulose membrane; the sample pad is arranged at the right end of the upper part of the gold label pad; an anti-haemophilus parasuis IgG is sprayed at the left end of the nitrocellulose membrane as a quality control line; a purified and renatured His-OppA fusion protein is sprayed at the right end of the nitrocellulose membrane as a detection line; and a colloidal gold-labeled purified and renatured His-OppA fusion protein is sprayed on the gold label pad. The haemophilus parasuis disease antibody detecting test strip can determine whether a haemophilus parasuis disease antibody exists in serum or not according to whether the detection line and the quality control line have color strips or not, and can detect all antibodies generated by a serotype haemophilus parasuis infection simply, conveniently and quickly.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
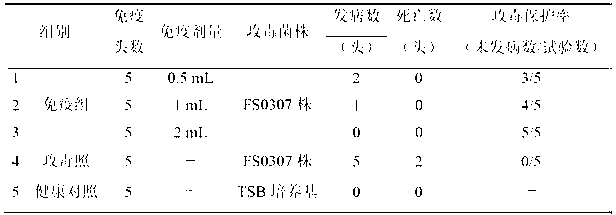
Serotype 4 haemophilus parasuis (HPs) vaccine strain
ActiveCN103194413AStable biological propertiesEpidemic preventionAntibacterial agentsBacteriaMortality rateSerotype
The invention relates to the field of vaccines of Haemophilus parasuis (HPs) in veterinary biological products and discloses a serotype 4 HPs vaccine strain. The class name of the vaccine strain is HPs. The strain number is FS0307. The vaccine strain has been collected in the China Center for Type Culture Collection, with collection number being CCTCC NO:M2013094 and collection date being March 21, 2013. The serotype 4 HPs strain FS0307 has stronger pathogenicity toward swine and has good immunogenicity. The inactivated vaccines prepared from the vaccine strain are safe and reliable and have quite good protective effects on the swine challenging homological strains. The morbidity and mortality of the immunized swinery are obviously reduced. Either the single vaccine or the combined vaccine has the immune effects equivalent or superior to the immune effects of the existing commercialized vaccines and can effectively prevent prevalence of HPs.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Formula of medicament for treating swine high fever and using method thereof
InactiveCN101732426AImprove survival rateRelief of immunosuppressionAmphibian material medical ingredientsAnthropod material medical ingredientsDiseaseAbdominal respiration
The invention relates to a formula of a medicament for treating swine high fever and a using method thereof. The medicament is prepared by dissolving 0.5 to 25 percent of Chinese angelica extract, 0.5 to 25 percent of rhubarb extract, 0.5 to 25 percent of Himalayan teasel root extract, 0.5 to 25 percent of common clubmoss herb extract, 0.5 to 25 percent of liquorice root extract, 0.5 to 25 percent of croton seed extract, 0.5 to 25 percent of toad venom, 0.5 to 25 percent of cicada slough extract, 0.5 to 25 percent of radix scutellariae extract, 0.5 to 25 percent of honeysuckle extract and 0.5 to 25 percent of common andrographis herb extract with solvent normal saline to obtain an injection, wherein the sum of the components and the balance is one hundred percent. The medicament has special effects on early and middle high fever, fulminant epidemic diseases from 2006 to 2009, mixed epidemic swine fever blue-eared disease of virus, pathogenic bacteria and worm, pseudorabies, blood worm, toxoplasmosis, lung plague, erysipelas, eircovims, streptococcus, haemophilus diseases, pneumonia, bronchitis and abdominal respiration and can relieve nose and mouth foaming in two hours.
Owner:孙胜俊
Haemophilus parasuis disease vaccine composition, preparation method and application thereof
InactiveCN104248755ASolve the problem of unsatisfactory effect of multivalent vaccineImprove immunityAntibacterial agentsBacterial antigen ingredientsDiseaseAntigen
The invention provides a Haemophilus parasuis disease vaccine composition, which contains an immune dose of capsule removed Haemophilus parasuis whole bacterium antigen and a veterinarily acceptable adjuvant. The capsule removed Haemophilus parasuis disease vaccine composition provided by the invention not only provides good immunoprotection to haemophilus parasuis of one same serotype, but also can have good preventive and therapeutic effects on Haemophilus parasuis diseases caused by currently epidemic Haemophilus parasuis of a variety of serotypes in China.
Owner:PU LIKE BIO ENG
Haemophilus parasuis (Hps) subunit vaccine composition and application
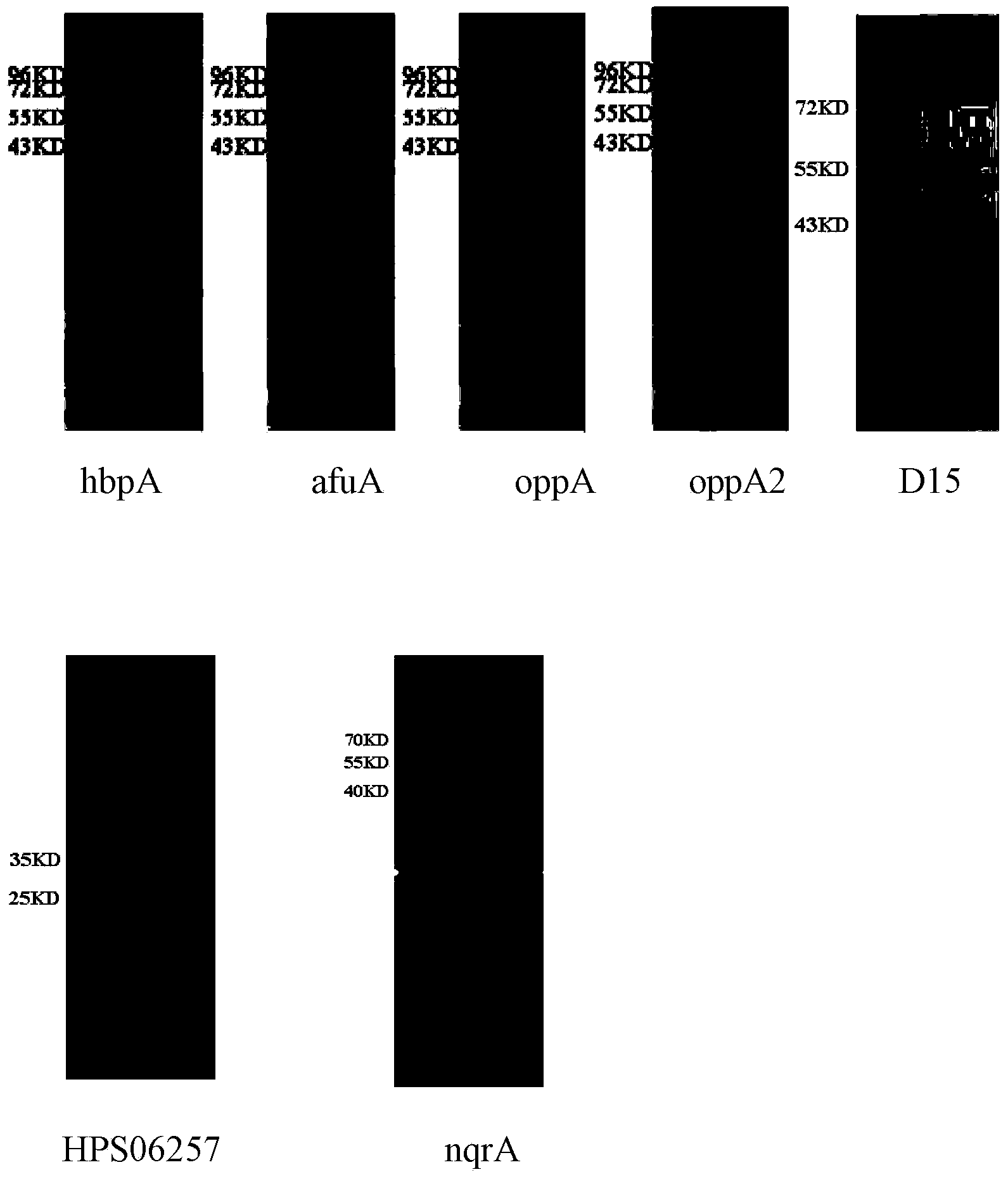
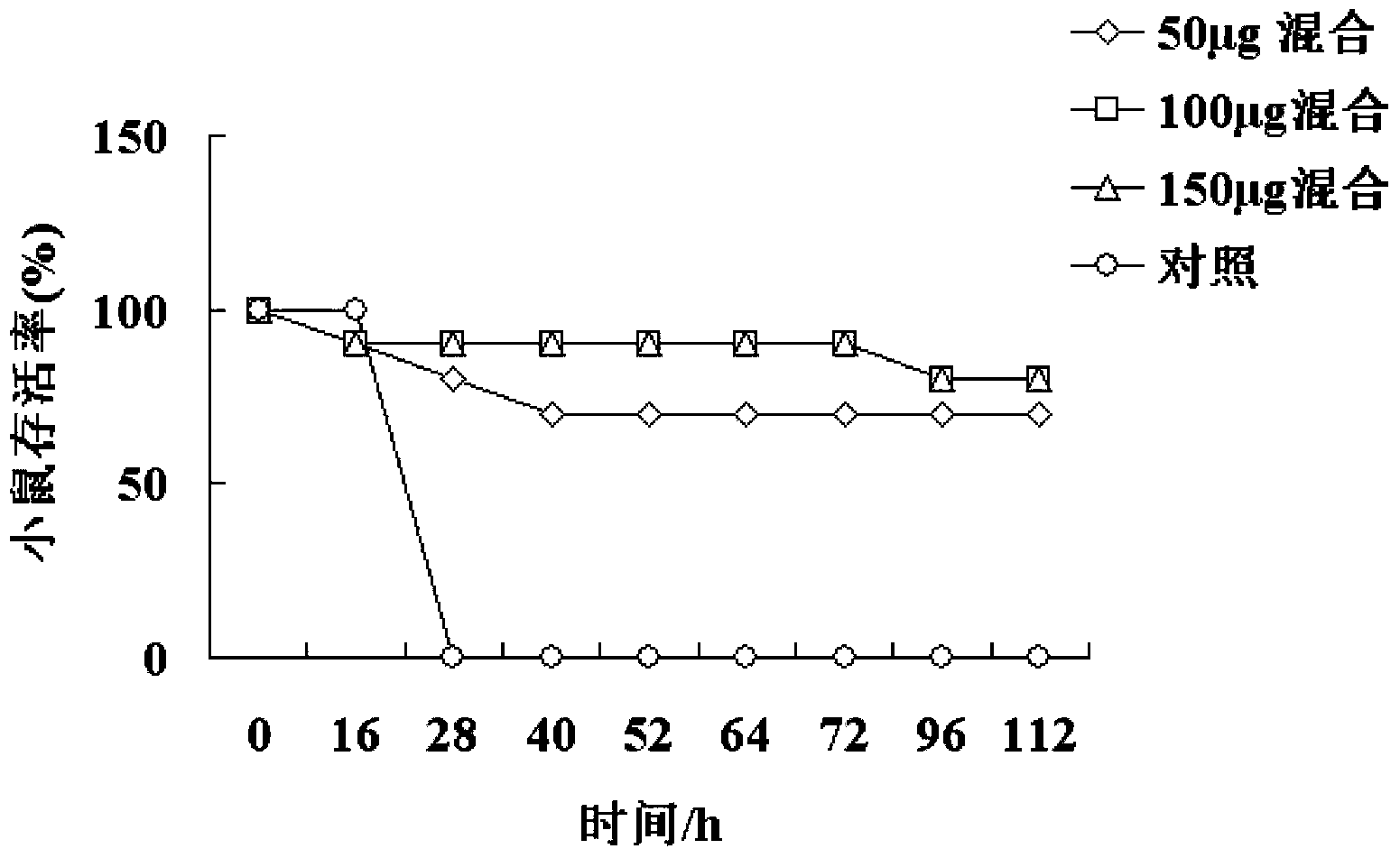
The invention provides a haemophilus parasuis (Hps) immunoprotective antigenic composition. The composition comprises seven haemophilus parasuis immunoprotective antigens, wherein the amino acid sequences of the seven haemophilus parasuis immunoprotective antigens are shown as SEQ ID NO:1-7 respectively. A plurality of new proteins with immunogenicity, including HbpA, afuA, oppA, oppA2, D15, Hps06257 and nqrA, are separated from the haemophilus parasuis (strain collection number: CVCC 3361), the nucleotide sequences of the proteins are shown as SEQ ID NO:8-14 in a sequence table, and 531, 346, 545, 513, 417, 263 and 448 amino acids are coded respectively. The coded products of the genes are new proteins with immunogenicity, and the mixture thereof can provide effective immunological protection for mice infected with haemophilus parasuis. The mixture of the immunogenic proteins of the recombinant haemophilus parasuis expressed by the composition has good safety and protection effect, and the immunological protection effect of the composition reaches 80 percent.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Preparation method of triple inactivated vaccine for pigs
InactiveCN104998256AImprove immunityHighlight immune functionAntibacterial agentsBacterial antigen ingredientsAntigenProtective antigen
The invention provides a preparation method of a triple inactivated vaccine for pigs. The method determines an antigen composition with excellent immunization effects by selection of the antigen. The prepared polyvalent vaccine has outstanding immunization effects. The prepared vaccine contains a PCP immunization protective antigen exotoxin (Aps), has cross immunization protection effects better than those of a whole cell inactivated vaccine, greatly reduces side reaction, and can simultaneously prevent haemophilus parasuis, swine streptococcosis and actinobacillus pleuropneumonia by combined immunization with inactivated haemophilus parasuis and streptococcus suis. Compared with haemophilus parasuis and streptococcus suis inactivated vaccines sold on the market, the triple inactivated vaccine has the same corresponding pathogen immune protection force. Compared with the actinobacillus pleuropneumonia inactivated vaccine sold on the market, the triple inactivated vaccine has a cross immunization protecting force on diseased pigs with different serotypes and realizes multiple protection purposes.
Owner:TIANJIN RINGPU BIO TECH
Indirect ELISA method for detecting haemophilus parasuis antibody by recombining Neu protein and kit of method
InactiveCN106501511AImprove accuracyAvoid false negativesBiological material analysisGlycosylasesSerotypeElisa method
The invention relates to the field of antibody detection, in particular to an indirect ELISA method for detecting a haemophilus parasuis antibody by recombining Neu protein and a kit of the method. The indirect ELISA method for detecting the haemophilus parasuis antibody by recombining the Neu protein comprises the steps that recombinant expressed and purified haemophilus parasuis Neuraminidase (Neu) protein serves as a coating antigen, accurate detection can be conducted on various serotype strains by optimizing the ELISA reaction condition, and the phenomenon that a large number of false negative results and false positive results appear can be better avoided; meanwhile, the phenomenon that the toxic risk exists in ELISA detection of a haemophilus parasuis disease can be effectively avoided, and the detection cost is greatly lowered.
Owner:永州职业技术学院
Application of serotype 5 haemophilus parasuis (HPs) vaccine strain
ActiveCN103191421AStable biological propertiesEpidemic preventionAntibacterial agentsAntibody medical ingredientsMortality rateHaemophilus Vaccines
The invention relates to the field of vaccines of Haemophilus parasuis (HPs) in veterinary biological products and discloses application of a serotype 5 HPs vaccine strain. The class name of the vaccine strain is HPs. The strain number is XX0306. The vaccine strain has been collected in the China Center for Type Culture Collection, with collection number being CCTCC No:M2013095 and collection date being March 21, 2013. The serotype 5 HPs strain XX0306 has stronger pathogenicity toward swine and has good immunogenicity. The inactivated vaccines prepared from the vaccine strain are safe and reliable and have quite good protective effects on the swine challenging homological strains. The morbidity and mortality of the immunized swinery are obviously reduced. Either the single vaccine or the combined vaccine has the immune effects equivalent or superior to the immune effects of the existing commercialized vaccines and can effectively prevent prevalence of HPs.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
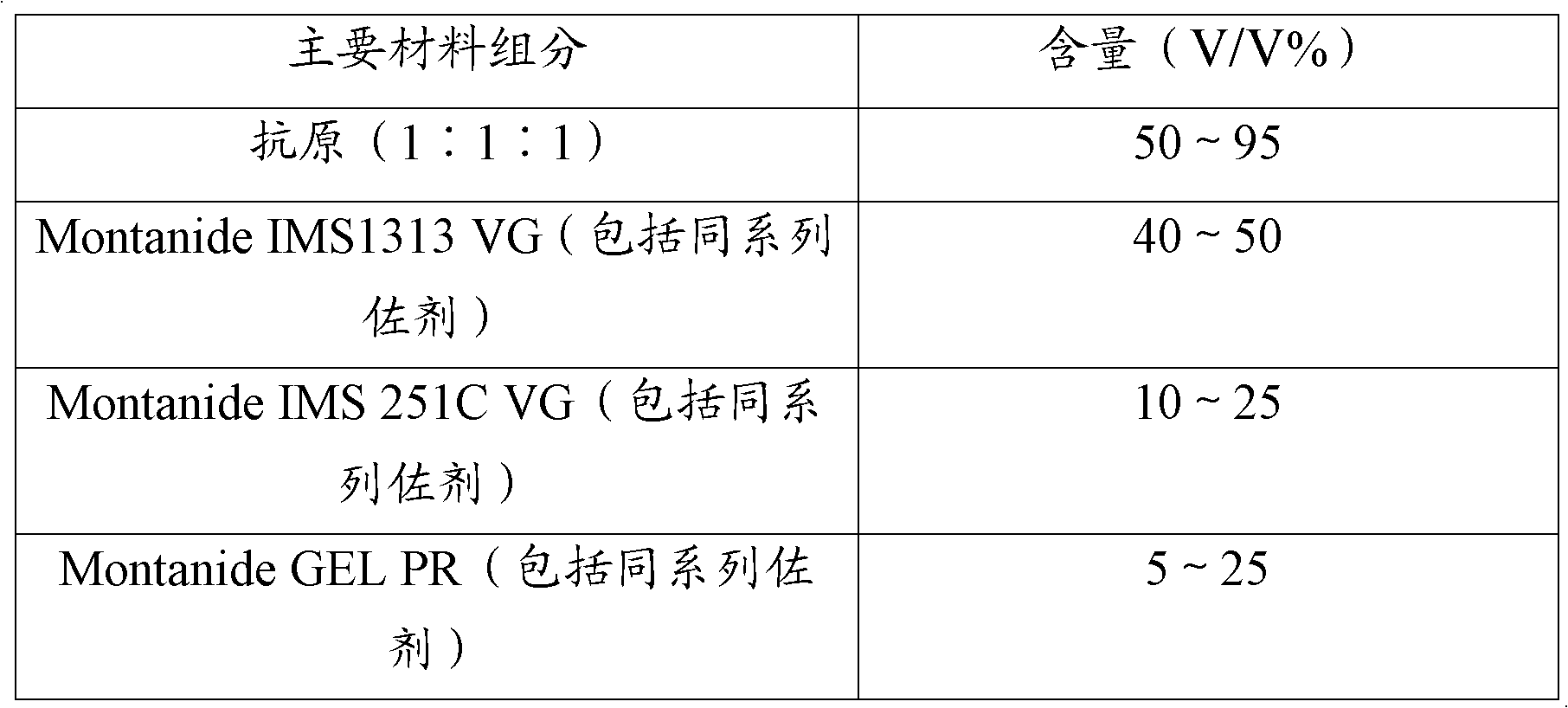
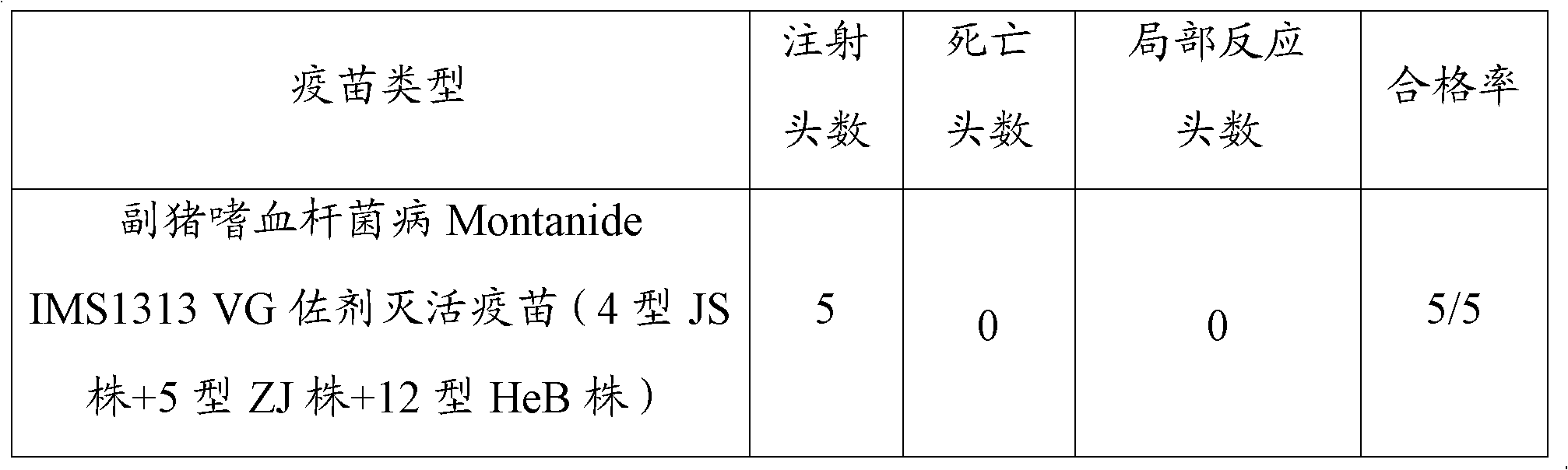
Haemophilus parasuis trivalent inactivated vaccine as well as production method and application thereof
InactiveCN108441446AImprove securityImprove immune efficiencyAntibacterial agentsBacterial antigen ingredientsDiseaseHaemophilus
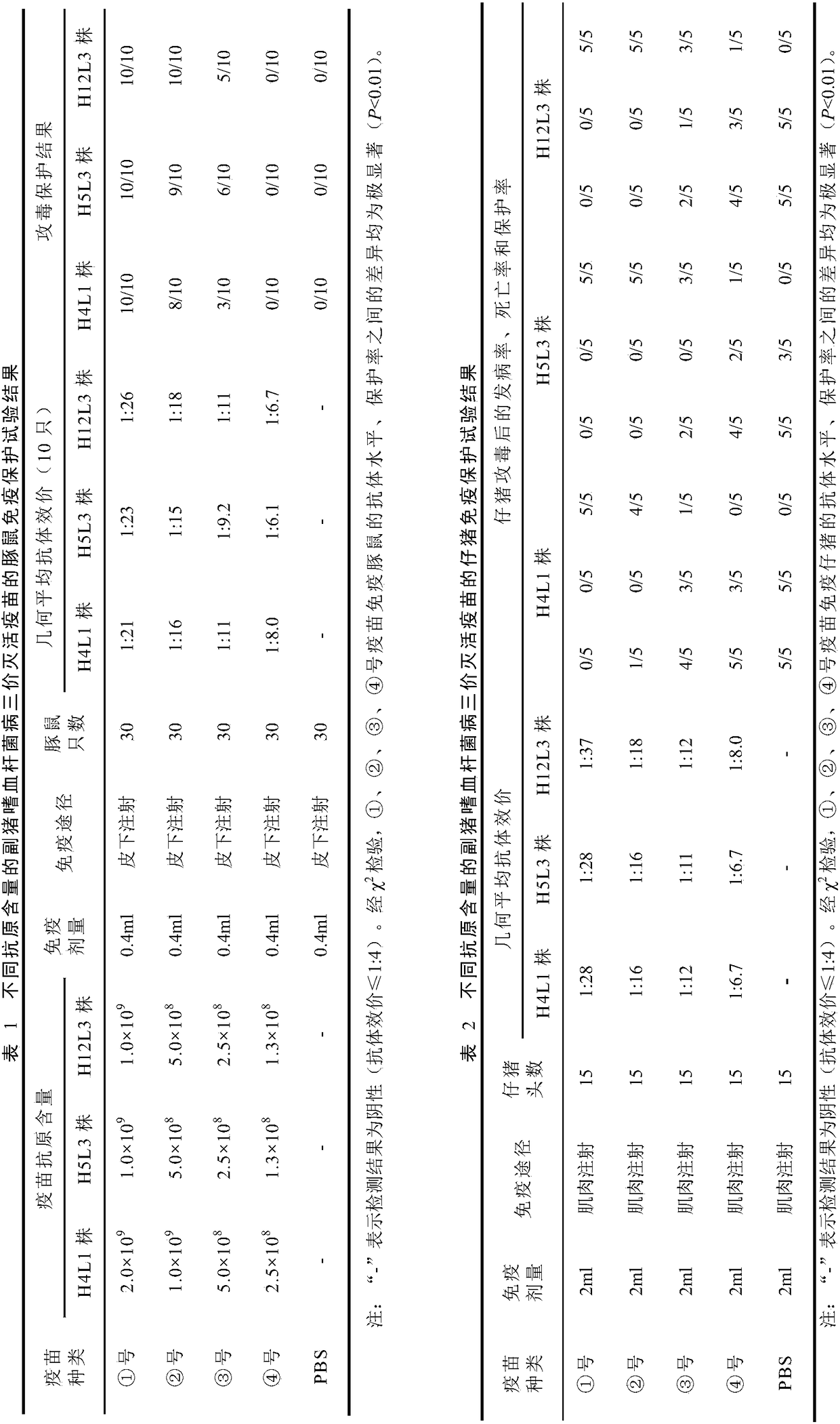
The invention discloses a haemophilus parasuis trivalent inactivated vaccine as well as a production method and application thereof. The haemophilus parasuis trivalent inactivated vaccine contains a haemophilus parasuis type-4 H4L1 strain, a type-5 H5L3 strain and a type-12 H12L3 strain being inactivated by a formaldehyde solution, as well as a water-based immunologic adjuvant, wherein the haemophilus parasuis type-4 H4L1 strain, the type-5 H5L3 strain and the type-12 H12L3 strain are all collected in the China Center for Type Culture Collection on 11 January, 2018 with the collection numbersof CCTCC M 2018019, CCTCC M 2018020 and CCTCC M 2018021 respectively. The trivalent inactivated vaccine disclosed by the invention is used for preventing the haemophilus parasuis disease caused by thetype-4, type-5 and type-12 haemophilus parasuis, and has the advantages of high security, high immune efficacy, and long immunity period and the like.
Owner:HENAN UNIV OF SCI & TECH +1
Swine streptococcicosis-Glasser's disease bivalent subunit vaccine and preparation method thereof
PendingCN110327460AReduce process complexityImprove immunityAntibacterial agentsBacterial antigen ingredientsAntigenDisease
The invention relates to a swine streptococcicosis-Glasser's disease bivalent subunit vaccine and a preparation method thereof. The vaccine includes Haemophilus parasuis antigen proteins AfuA, OppA2,CdtB and OppA, and Streptococcus suis antigens which are MRP and SLY; or includes a Haemophilus parasuis fusion protein AfuA-OppA2 and a fusion protein CdtB-OppA which are two antigens, and Streptococcus suis antigen proteins MRP and SLY which are two antigens. The vaccine can stimulate a strong immune response in mice, has good cross-protection effect on mice conteracting different serotypes of Haemophilus parasuis and Streptococcus suis, and is superior to traditional inactivated vaccines. The subunit vaccine provides basis for development and research of efficient, broad-spectrum, and inexpensive swine streptococcicosis-Glasser's disease vaccines.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
Combined inactivate vaccine for haemophilus parasuis disease and streptococcus suis disease and preparation method for same
ActiveCN103157101ALittle side effectsGood immune securityAntibacterial agentsBacterial antigen ingredientsDiseaseAntigen
The invention relates to a combined inactivate vaccine for haemophilus parasuis disease and streptococcus suis disease and a preparation method for the same. The vaccine is prepared from inactivated haemophilus parasuis serotype-4 JS strain, haemophilus parasuis serotype-5 ZJ strain, streptococcus suis serotype-2 colony-R SC strain and streptococcus suis colony-C streptococcus equisubsp zooepidemius which are used as antigens, and adjuvants which are acceptable in veterinary medicine, and capable of performing immunoprophylaxis on the haemophilus parasuis disease caused by haemophilus parasuis, the streptococcus suis disease caused by streptococcus suis, and the mixed infection of haemophilus parasuis and streptococcus suis simultaneously. The vaccine is low in side effects, free from endotoxin and impure proteins in serum, good in immune safety, capable of achieving the effect of preventing multiple diseases by one injection in clinical and thus reducing cost, and capable of meeting the requirements of different users.
Owner:PU LIKE BIO ENG
Veterinary compound enrofloxacin injection and preparation method thereof
InactiveCN101658526APromote absorptionEfficient drug deliveryAntibacterial agentsAntipyreticFLUNIXIN MEGLUMINETrimethoprim
The invention relates to a veterinary compound enrofloxacin injection and a preparation method thereof. The veterinary compound enrofloxacin injection mainly comprises enrofloxacin, sulfamethoxazole,trimethoprim, flunixin meglumine, tylosin, organic solvent and water for injection. The veterinary compound enrofloxacin injection is used as a veterinary special compound preparation, used for treating various infectious diseases of a respiratory system, a digestive system and a skin soft tissue caused by livestock and poultry bacterial and mycoplasma infection, particularly has special effects on secondary hemophilus disease of pigs and poultries caused by hemophilus and has the advantages of convenient use, short course, low drug resistance and the like.
Owner:陈建波
Preparation method of novel adjuvant for haemophilus parasuis disease inactivated vaccines
PendingCN105833267AImprove Hps antibody titerIncrease IL-2, IL-4 levelsAntibacterial agentsAntibody medical ingredientsPhagocyteAntigen
The invention relates to a preparation method for a novel adjuvant for haemophilus parasuis disease inactivated vaccines .The method comprises the following steps of 1, separating and extracting bacterial strains and preparing bacteria solution antigen; 2, preparing a vaccine stock solution; 3, preparing finished vaccines, wherein a ginkgo biloba extract is selected as an adjuvant, and ginkgo biloba polyvalent inactivity vaccines of different concentrations, components and contents are prepared and obtained .The effective components of polysaccharose substances of the adjuvant are prepared through separation and extraction of traditional Chinese herbal medicine ginkgo biloba plants and then combined with haemophilus parasuis polyvalent inactivity vaccines, and the haemophilus parasuis ginkgo biloba adjuvant inactivated vaccines are prepared .The inactivated vaccines have the good immune modulating function, can regulate lymphocyte and phagocyte activity, can regulate the cell factor level in body serum, and have the advantages of being natural, low in toxicity, free of drug residues and the like .
Owner:LONGYAN UNIV
Anti-atrophic rhinitis and haemophilus parasuis vaccine composition and preparation thereof
ActiveCN103816536AChange cognitive biasImprove immunityAntibacterial agentsBacterial antigen ingredientsDiseaseBordetella bronchiseptica antigen
The invention relates to an anti-atrophic rhinitis and haemophilus parasuis vaccine composition and a preparation method thereof. The vaccine composition contains components such as a pig bordetella bronchiseptica antigen, a pig toxigenic pasteurellamultocida antigen and a haemophilus parasuis antigen, wherein a bacterium solution of each antigen component is obtained by a fermentation cultivation method, and is concentrated by an ultrafiltration concentration technology. According to the vaccine composition, the antigens in the vaccine composition are high in content and comprehensive, so that animals can be comprehensively and effectively protected; a hydrogel adjuvant is adopted, so that the vaccine composition can be well absorbed, and is quick in immune response, a local immune part is free of adverse reaction, and the immune protection of pigs can be improved; the problem of effective and comprehensive protection of the pigs during mixed infection is effectively solved, immunization procedures are simplified, the effect of preventing multiple diseases by once injection is achieved, side effects caused by multiple immunization are reduced, the immunization cost is lowered, and the vaccine composition is economical and practical.
Owner:PU LIKE BIO ENG
Vaccine composition containing porcine circovirus type 2 antigen and haemophilus parasuis antigen and preparation method and application thereof
The invention relates to a multi-vaccine composition for pigs, in particular to a vaccine composition resisting porcine circovirus type 2 (PCV2) infection and haemophilus parasuis (HPS) infection at the same time. The vaccine composition is prepared from at least one kind of a porcine circovirus type 2 antigen, at least one kind of a haemophilus parasuis antigen, carriers, excipients and adjuvants, wherein the carriers, the excipients and the adjuvants can be obtained in the veterinary medicine field. The vaccine composition can be used for preventing and treating porcine circovirus type 2 related diseases and haemophilus parasuis diseases.
Owner:PULIKE BIOLOGICAL ENG INC
Vaccine composition, and preparation method and application thereof
ActiveCN104288762ALow costReduce the number of vaccinationsAntibacterial agentsAntiviralsDiseaseHaemophilus
The invention provides a vaccine composition. The vaccine composition contains an immune amount of classical swine fever virus antigens, an immune amount of Haemophilus parasuis antigens, an immune amount of type 2 porcine circovirus antigens and a veterinarily acceptable carrier. The vaccine composition can effectively prevent and treat swine fever, the porcine circovirus disease and the Haemophilus parasuis disease, can reach double single vaccine injection immunization effect through one time injection, and has the advantages of few side reactions, long immune period, short time and less labor consumption.
Owner:PU LIKE BIO ENG
Serotype 7 haemophilus parasuis natural weak virulent strain and application thereof
ActiveCN108018230AReduce pathogenicityIncrease productivityAntibacterial agentsBacteriaSerum igeHaemophilus
The invention discloses a serotype 7 haemophilus parasuis natural weak virulent strain and application thereof. The serotype 7 haemophilus parasuis natural weak virulent strain is deposited at China Center for Type Culture Collection on November 22, 2017, and the accession number is CCTCC M 2017706. The serotype 7 haemophilus parasuis natural weak virulent strain has very low pathogenicity and issuitable for preparation of a medicament for treatment of haemophilus parasuis, and the medicament is a vaccine. A preparation method of the vaccine includes the following steps: S1, thallus culture;S2, thallus collection; S3, freeze-drying. Inactivation and inactivation inspection are not needed in the preparation of the medicament, the preparation method is simple, and production efficiency ofthe medicament is higher. When the vaccine is applied to 28 to 35-day-old piglets in immunizing dose of 2.0*108CFU / mL, the immune protection rate is 100%.
Owner:HUAZHONG AGRI UNIV
Method for preparing bacterial ghost vaccine of haemophilus parasuis as well as product and application thereof
InactiveCN103446581ARepress transcriptionImprove cracking efficiencyAntibacterial agentsMicroorganism based processesEPROMHaemophilus
The invention discloses a method for preparing a bacterial ghost vaccine of haemophilus parasuis as well as a product and application thereof. The method comprises the following steps of connecting a mutational bacteriophage splitting gene E Eprom (as shown in SEQ ID No:1) with pBV220 to obtain an efficient splitting plasmid vector pBV-Eprom; converting the pBV-Eprom into haemophilus parasuis, propagating at 37 DEG C, and inducing the Eprom gene to express at 42 DEG C, and collecting the product which is unexpressed finally to obtain haemophilus parasuis bacterial ghost, wherein the Eprom is obtained by carrying out mutation on a promoter region of the bacteriophage splitting gene E, and the temperature for culturing bacteria is changed to 37 DEG C from the existing 28 DEG C by the Eprom; moreover, the splitting efficiency is high, the initial induced concentration and the large-scale production capacity are high, and the culture-splitting efficiency of a fermentation tank is as high as 99.99995%. The bacterial ghost vaccine of the haemophilus parasuis disclosed by the invention has good safety and immune protective efficacy, can be used for stimulating a body to generate a high-titration antibody, and also can be used for providing good cross immune protection for attack of a virulent strain of the haemophilus parasuis with different serotypes.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Application of serum 4 type haemophilus parasuis vaccine strain
ActiveCN103182077AStable biological propertiesEpidemic preventionAntibacterial agentsAntibody medical ingredientsSerum igeHaemophilus
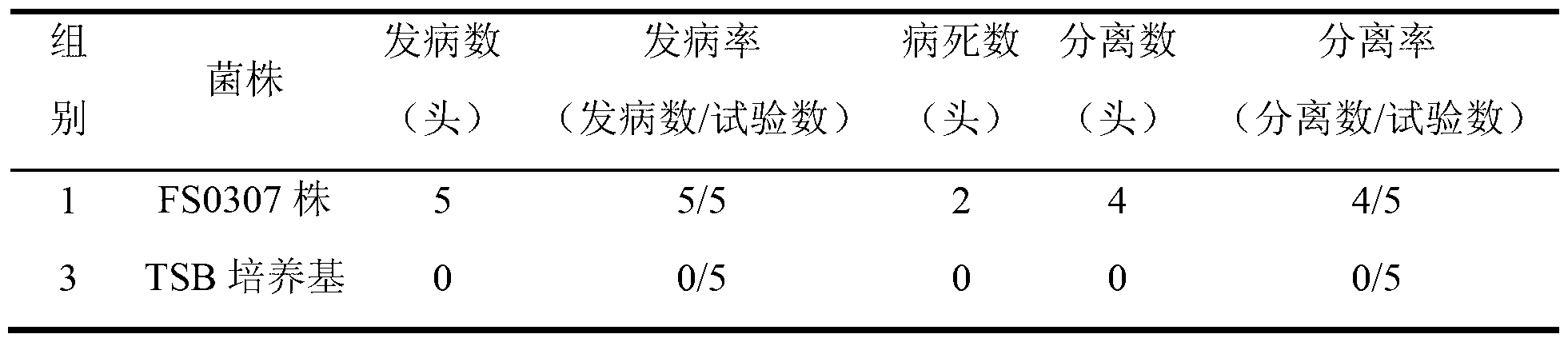
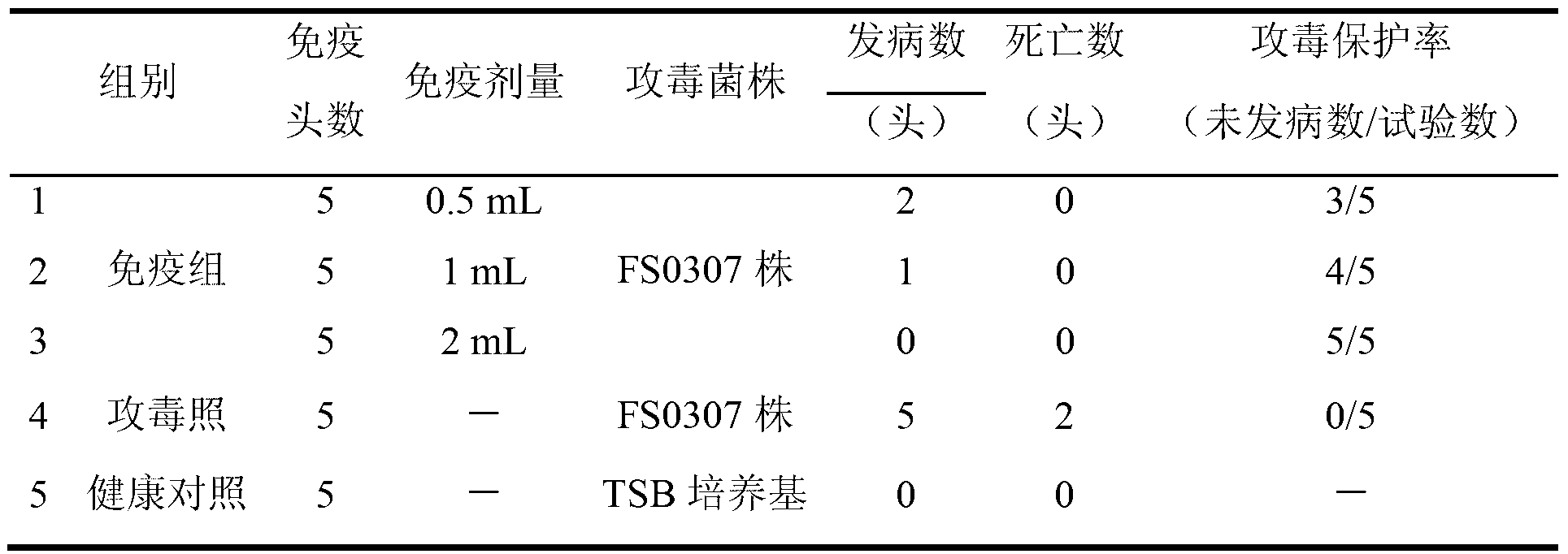
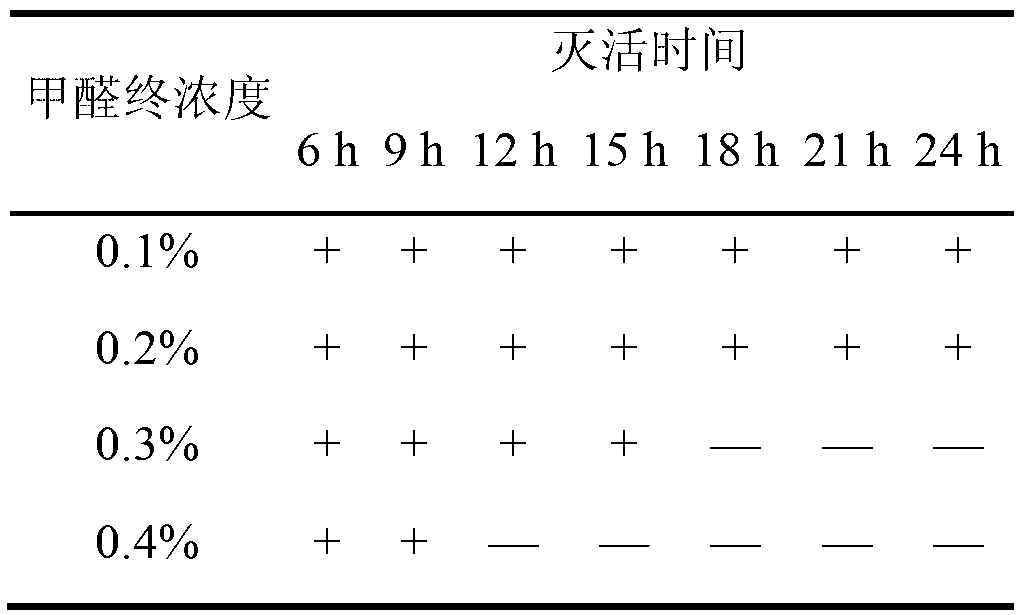
The invention relates to the field of haemophilus parasuis vaccine strains in veterinarian biological products and discloses an application of a serum 4 type haemophilus parasuis strain. The serum 4 type haemophilus parasuis strain is classified and named as haemophilus parasuis with the strain number of FS0307 and is preserved in China Center for Type Culture Collection with the preservation number of CCTCC NO:M2013094 and the preservation date of March 21, 2013. The serum 4 type haemophilus parasuis strain FS0307 has strong pathogenicity to pigs and good immunogenicity; an inactivated vaccine prepared by applying the serum 4 type haemophilus parasuis strain is safe and reliable and has a good protection effect to pigs attacked by toxicity of homogenous strains; and morbidity and mortality of the pigs after immunization are obviously reduced. Whether a single vaccine or a combined vaccine, immunity of the serum 4 type haemophilus parasuis strain achieves or is better than that of the existing commercialization vaccine on the market, and prevalence of haemophilus parasuis can be effectively prevented.
Owner:JIANGSU ACAD OF AGRI SCI
Haemophilus parasuis indirect hamagglutination detection reagent
The invention relates to the field of veterinary detection reagents, and discloses a haemophilus parasuis indirect hamagglutination detection reagent. The haemophilus parasuis indirect hamagglutination detection reagent is prepared by taking multiple serotype haemophilus parasuis whole protein mixtures as antigens, and is capable of rapidly and sensitively detecting serum antibodies of all serotype haemophilus parasuis diseases. The production technology is optimized through balanced mixing of each whole protein antigen, and then sensibilization and double hydroformylation of mutton red cells; and the prepared haemophilus parasuis indirect hamagglutination detection reagent is stable, sensitive, good in specificity, long in storage life, simple in operation, and applicable to a large amount of clinical sample detection on the haemophilus parasuis diseases, immune level detection and epidemiology investigation.
Owner:JIANGSU ACAD OF AGRI SCI
Traditional Chinese veterinary medicine compound preparation Shuanghuanglian for injection as well as preparation technology and application thereof
InactiveCN103585280AReduce use costMature and stable processAntibacterial agentsPowder deliveryCelluloseSwine plague
The invention relates to a traditional Chinese veterinary medicine compound preparation Shuanghuanglian for injection as well as a preparation technology and an application thereof. The compound preparation comprises the components in parts by weight as follows: 1300-1700 parts of honeysuckle, 1300-3700 parts of radix scutellariae and 2500-7500 parts of fructus forsythiae. Slow-release accessories comprise 150 parts of hydroxymethyl propyl cellulose and 100 parts of sodium carboxymethyl cellulose. The compound preparation contains the slow-release accessories, so that the injection time of large animals can be reduced, and the stress response of the large animals can be reduced. The traditional Chinese veterinary medicine compound preparation for injection has antiviral, antibacterial, anti-inflammatory, antipyretic, analgesic, immunity-improving effects and the like. Veterinarian clinic proves that the compound preparation can be used for treating epidemic diarrhea; transmissible gastroenteritis; necrotic enteritis; septicemia caused by various epidemic diseases; cough, dyspnea, skin cyanosis, arthrocele which are caused by chromic respiratory diseases such as streptococcosis, haemophflussuis, contagious porcine pleuropneumonia, swine plague and the like; and porcine reproductive and respiratory syndrome and cow mastitis.
Owner:QINGDAO AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com