Preparation method of novel adjuvant for haemophilus parasuis disease inactivated vaccines
A technology for haemophilus suis disease and inactivated vaccines, which is applied in the field of preparation of new adjuvants for haemophilus parasuis inactivated vaccines, can solve the problems of increasing difficulty and the influence of propolis adjuvant application and promotion, and achieve the improvement of antibody titer Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] The preparation method of the new adjuvant of Haemophilus parasuis inactivated vaccine of the present invention comprises the following steps:
[0021] (1) Separating and extracting bacterial strains and preparing bacterial fluid antigens;
[0022] (2) Prepare vaccine stock solution;
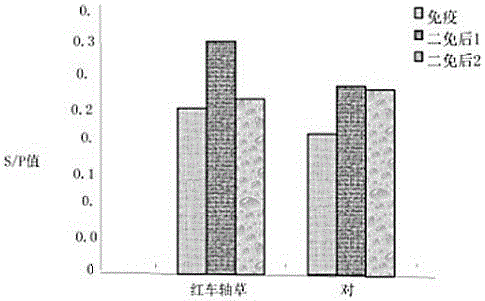
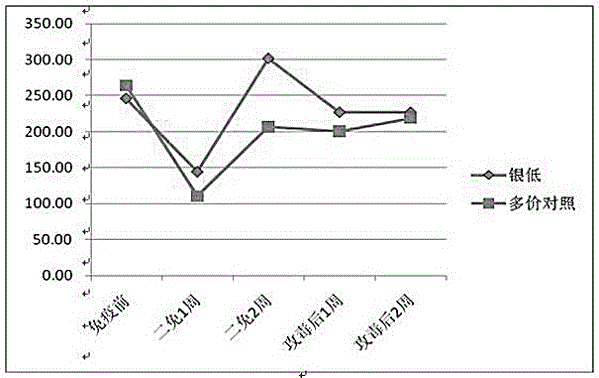
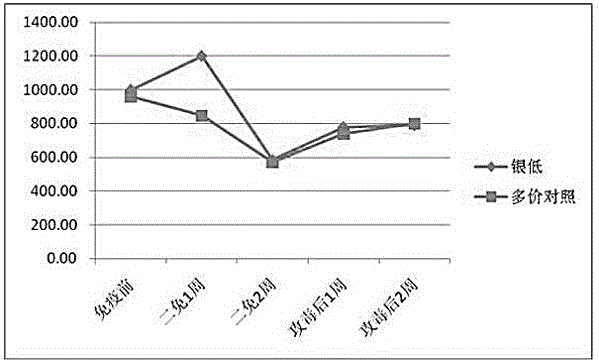
[0023] (3) Preparation of finished vaccine products: Ginkgo biloba extract was selected as an adjuvant to prepare ginkgo biloba polyvalent inactivated vaccines with different concentrations of ingredients.
[0024] In the immunization dose of 2 mL / head, the amount of adjuvant is 2.5 mL-12.5 mL.
[0025] Ginkgo biloba extract stock solution contains 40 mg of extract per ml, which contains 9.6 mg of ginkgo flavonoid glycosides and 2.4 mg of terpene lactones (gingkolide, bilobalide).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com