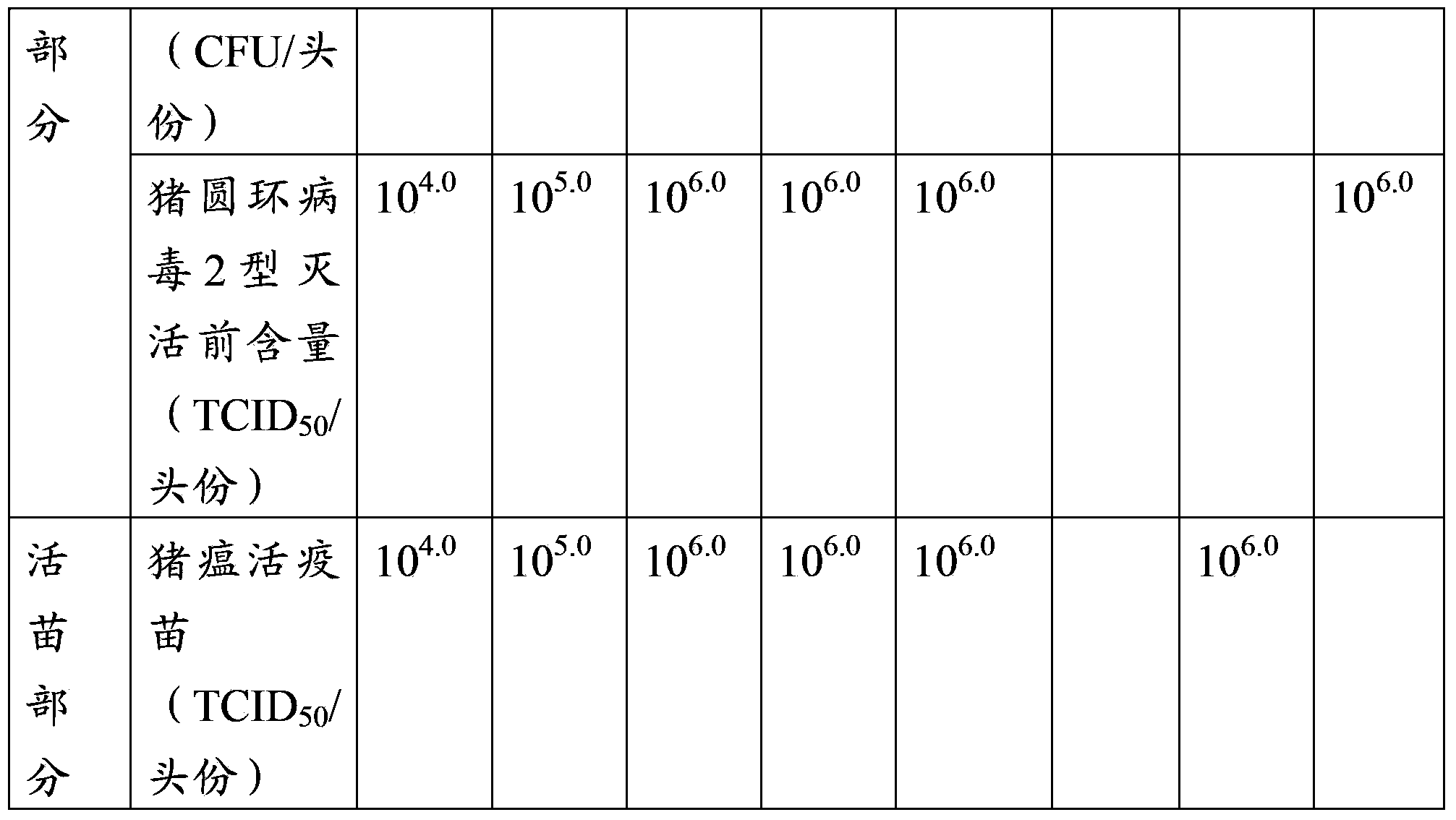Vaccine composition, and preparation method and application thereof
A vaccine composition and antigen technology, which is applied in the field of vaccine compositions, can solve the problems of triple combination vaccine, increased intensity, stress response of pig herds, etc., for which there is no Haemophilus parasuis for swine fever, so as to reduce costs and inoculations. The number of times, the effect of boosting the immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Preparation of swine fever antigen, porcine circovirus antigen and Haemophilus parasuis antigen
[0040] 1 Preparation of swine fever antigen
[0041] 1.1 Use ST cells (purchased from ATCC) that are highly sensitive to classical swine fever virus (swine fever lapinized attenuated strains are preserved by the China Veterinary Drug Supervision Institute, with the preservation number AV1412) containing 0.125% pancreatin and 0.03% The digestion solution of EDTA is digested and dispersed. After the cell count, the cell culture flask is inoculated, and 1.5%-5% FBS MEM cell culture solution is added. At the same time, the seed poison is added according to the MOI=0.01-0.6 ingestion dose. The more preferred dose is MOI=0.1-0.6, and it is more preferable that the poisoning dose is MOI=0.2-0.4, and it is cultured in a 34-37°C incubator, and further preferably the culture temperature is 34-35°C.
[0042] After three days of culture, the first collection of poisons is carried o...
Embodiment 2
[0059] Example 2 Preparation of a triple mixed vaccine composition of swine fever, swine ring and Haemophilus parasuis
[0060] Preparation of 1 inactivated vaccine against Haemophilus suis and porcine ring
[0061] 1.1 Preparation of preservatives
[0062] 1% (w / v) thimerosal aqueous solution: 1g thimerosal dissolved in 100ml purified water, autoclaved at 121°C for 30 minutes for later use.
[0063] 1.2 Preparation of diluent
[0064] Sterile PBS buffer solution: Dissolve 8g sodium chloride, 0.25g potassium chloride, 3.63g disodium hydrogen phosphate, 0.24g potassium dihydrogen phosphate in 900ml purified water, then dilute to 1L, autoclave at 121°C for 30min spare.
[0065] 1.3 Vaccine adjuvant treatment
[0066] Gel01 adjuvant sterilization: transfer Gel01 adjuvant into a sterilizable container, autoclave at 121°C for 30 minutes for later use.
[0067] 1.4 Matching seedlings
[0068] After aseptic operation, the concentrated Haemophilus parasuis antigen, porcine circovirus type 2 antige...
Embodiment 3
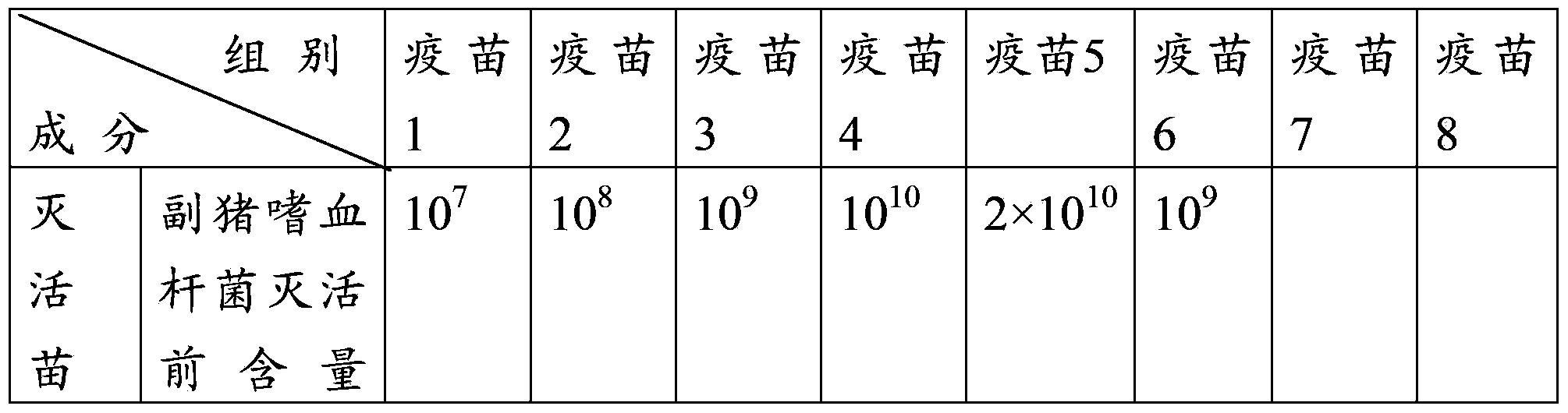
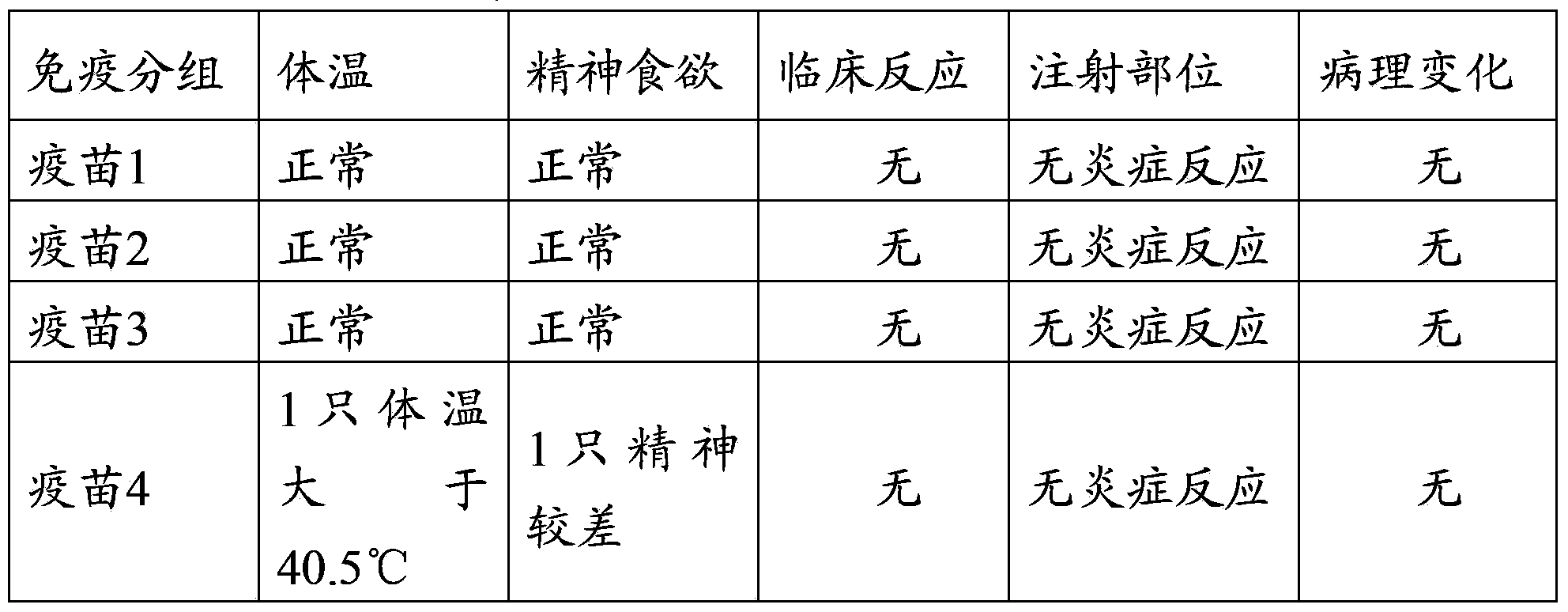
[0076] Example 3 Efficacy test of the triple mixed vaccine of swine fever, pig circle and Haemophilus parasuis with different antigen content
[0077] 1 Test materials
[0078] Vaccine 1 prepared in Example 2 (Haemophilus parasuis 10 7 CFU / tou, swine fever virus 10 4.0 TCID 50 / Toufen, Porcine Circovirus 10 4.0 TCID 50 / Head), vaccine 2 (Haemophilus parasuis 10 8 CFU / tou, swine fever virus 10 5.0 TCID 50 / Toufen, Porcine Circovirus 10 5.0 TCID 50 / Head), vaccine 3 (Haemophilus parasuis 10 9 CFU / tou, swine fever virus 10 6.0 TCID 50 / Toufen, Porcine Circovirus 10 6.0 TCID 50 / Head), vaccine 4 (Haemophilus parasuis 10 10 CFU / tou, swine fever virus 10 6.0 TCID 50 / Toufen, Porcine Circovirus 10 6.0 TCID 50 / Head) and vaccine 5 (Haemophilus parasuis 2×10 10 CFU / tou, swine fever virus 10 6.0 TCID 50 / Toufen, Porcine Circovirus 10 6.0 TCID 50 / Toufen).
[0079] Weaned piglets at the age of 3-4 weeks without swine fever, pig ring and Haemophilus parasuis antibodies.
[0080] 2 Test metho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com