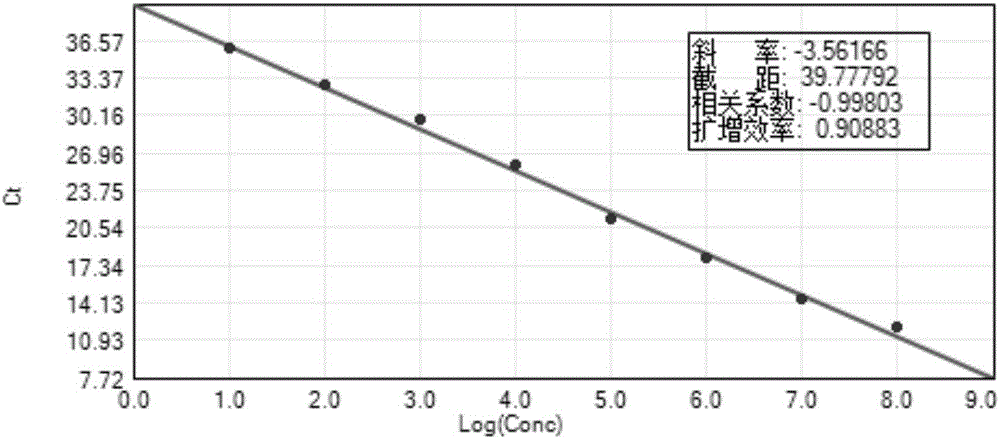
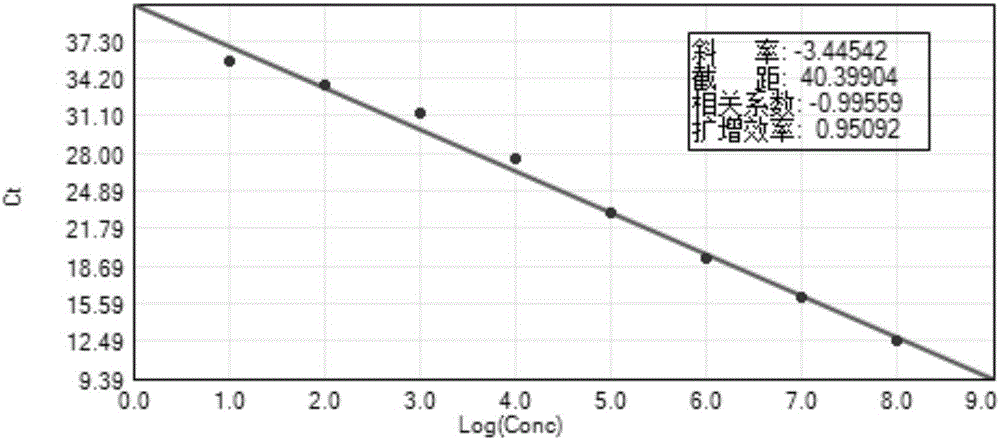
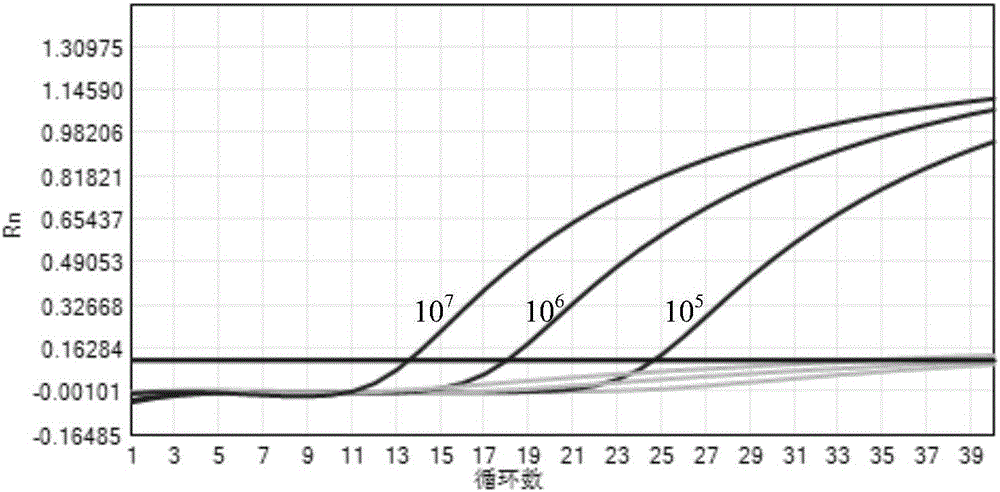
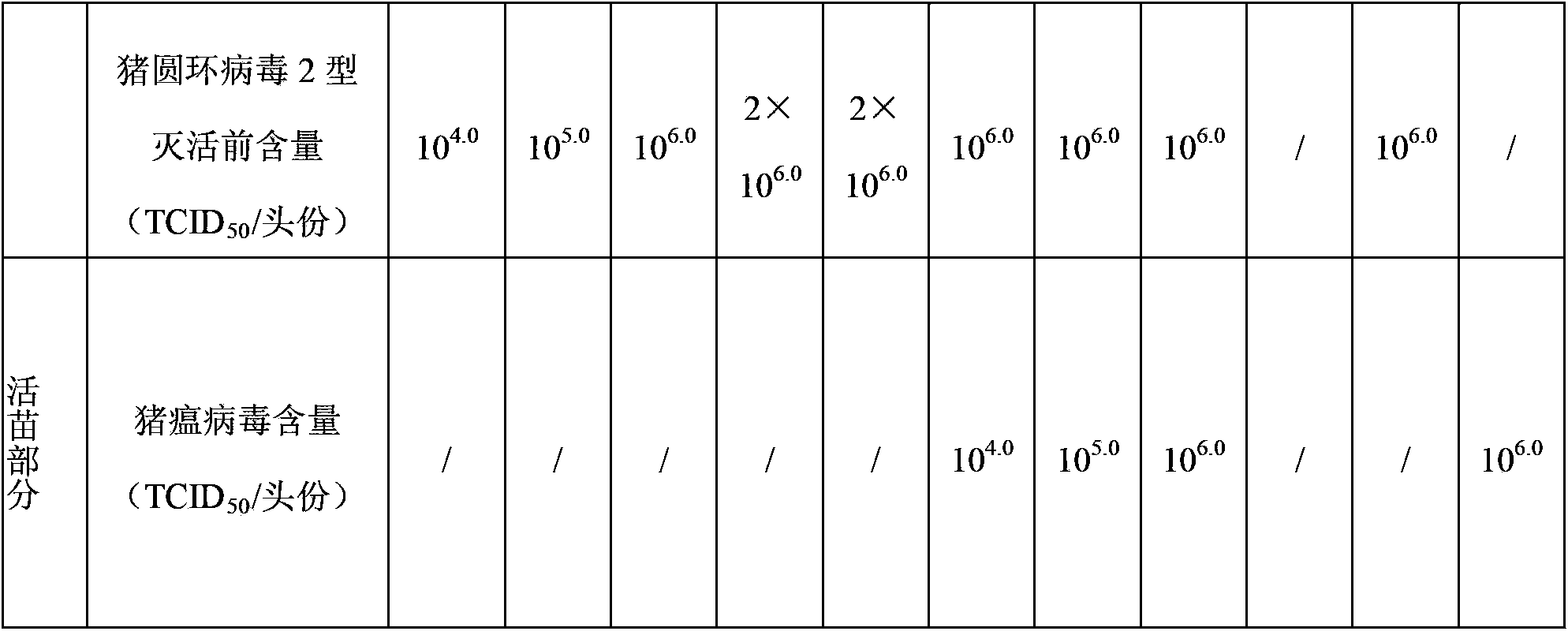Patents
Literature
117 results about "Henipavirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Henipavirus is a genus of RNA viruses in the family Paramyxoviridae, order Mononegavirales containing five established species. Henipaviruses are naturally harboured by pteropid fruit bats (flying foxes) and microbats of several species. Henipaviruses are characterised by long genomes and a wide host range. Their recent emergence as zoonotic pathogens capable of causing illness and death in domestic animals and humans is a cause of concern.
Taqman-MGB fluorescent quantitative PCR kit and method for detecting 12 common viruses and bacteria of pig at same time
ActiveCN105624330AQuick checkSensitive detectionMicrobiological testing/measurementPorcine reproductive and respiratory syndrome virusPorcine circovirus
The invention provides a Taqman-MGB fluorescent quantitative PCR kit and a method for detecting 12 common viruses and bacteria of pigs at the same time. The kit comprises PCR reaction liquids A / B / C, wherein the PCR liquids comprise primer pairs and Taqman probes for porcine parvovirus (PPV), type-II streptococcus suis (SS-II), a porcine pseudorabies virus (PRV), type-II porcine circovirus (PCV-2), a hog cholera virus (CSFV), a pig foot and mouth disease virus (FMDV), a porcine reproductive and respiratory syndrome virus (PRRSV), a high pathogenicity porcine reproductive and respiratory syndrome virus strain (Hp-PRRSV), a transmissible gastroenteritis virus (TGEV), an epidemic diarrhea virus (PEDV), rotavirus (PRTV) and a swine influenza virus (SIV) respectively. 12 pathogens of pigs can be detected rapidly and effectively at the same time, the detection method is high in accuracy, specificity and sensitivity and is good in stability, and rapid diagnosis and effective detection on pathogens to be detected can be achieved.
Owner:BEIJING YISEN BIOTECH
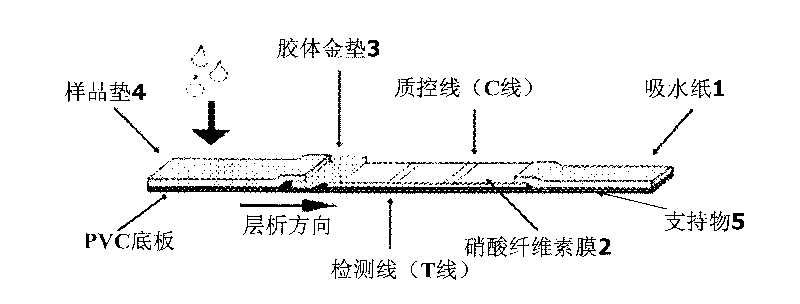
Colloidal gold immunochromatographic test strip for detecting wild-type classical swine fever virus
The invention discloses a colloidal gold immunochromatographic test strip for detecting wild-type classical swine fever virus, which consists of water absorbent paper (1), a cellulose nitrate membrane (2), a colloidal gold pad (3), a sample pad (4) and a support (5), wherein the cellulose nitrate membrane contains a detection line which is formed by coating monoclonal antibody HQ06 of anti-classical swine fever virus E2 protein and a quality control line which is formed by coating rabbit anti-mouse IgG antibody; and the colloidal gold pad is combined with colloidal gold-labeled monoclonal antibody 6E10 of the anti-classical swine fever virus E2 protein. The test strip does not react with C-strain of classical swine fever virus, bovine viral diarrhea virus, porcine reproductive and respiratory syndrome virus, transmissible gastroenteritis virus, porcine epidemic diarrhea virus, porcine rotavirus, pseudorabies virus, porcine parvovirus and porcine circovirus type 2, and can accurately and sensitively identify the wild-type classical swine fever virus, thereby having good specificity, sensitivity and repeatability.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
ELISA kit for distinctively detecting antibodies of classical swine fever (CSF) vaccine immunity and wild virus infection and preparation method thereof
InactiveCN101900731AConvenient prevention and controlImprove purification effectMaterial analysisElisa kitStructural protein
The invention relates to an ELISA kit for distinctively detecting antibodies of classical swine fever (CSF) vaccine immunity and wild virus infection and a preparation method thereof. An indirect ELISA kit or a blockage ELISA kit is formed by expressing and purifying classical swine fever virus (CSFV) non-structural protein NS3, coating a solid-phase carrier and assembling with other matched reagents. The kit has the characteristic of distinctively detecting different antibodies generated by the CSF vaccine immunity and the wild virus infection. The kit can distinctively diagnose the CSF vaccine immunity and the wild virus infection.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
PCR primer for detecting African swine fever virus, kit and application thereof
ActiveCN105695634AAccurate diagnosisEasy to distinguishMicrobiological testing/measurementDNA/RNA fragmentationAfrican swine feverNucleotide sequencing
The invention discloses a PCR primer for detecting an African swine fever virus, a kit and application thereof, and belongs to the detection field of the African swine fever virus. According to a conserved region of an ASFV p72 gene on the GenBank, four pairs of primers are designed, the primer with strong specificity and high sensitivity is screened out from the four pairs of primers, and the primer is composed of nucleotide sequences as shown in SEQ ID No.1 and SEQ ID No.2. The invention further discloses a kit prepared from the primer and used for detecting the African swine fever virus, and a corresponding PCR detection method is established. The detection method established by the invention is more specific and sensitive in comparison with two African swine fever virus PCR detection methods recommended by OIE; and the clinical sample detection result shows that the PCR detection method disclosed by the invention is simple in operation, low in cost, good in specificity and high in sensitivity, and can be effectively applied to the screening and fast diagnosis of the African swine fever.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Preparation method and application of classical swine fever virus recombinant subunit vaccine
InactiveCN104826100ANo risk of contaminationImprove securityAntiviralsAntibody medical ingredientsProtein targetVaccine Production
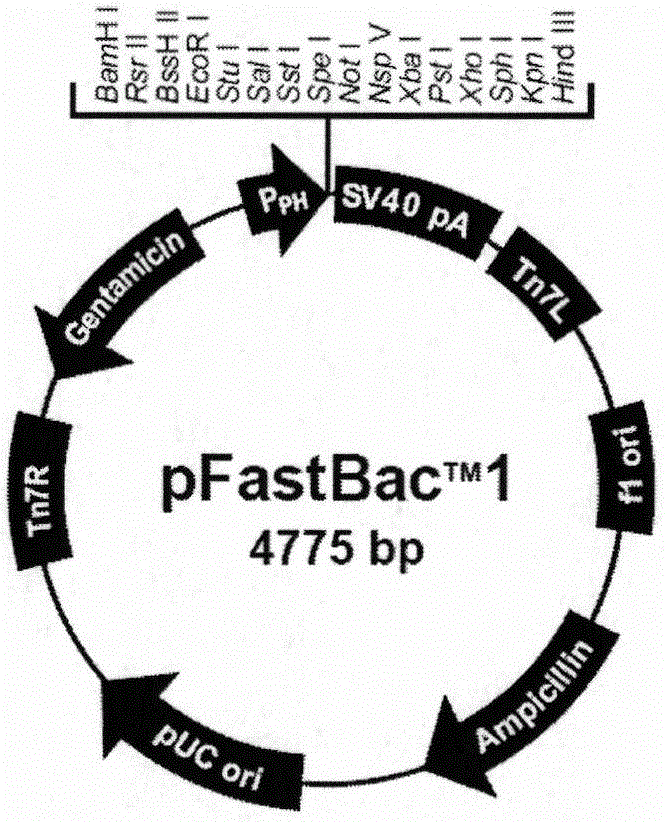
The invention discloses a preparation method and application of a classical swine fever virus recombinant subunit vaccine with the amino acid sequence shown as SEQ ID No.1. The preparation method of the classical swine fever virus recombinant subunit vaccine typically includes the following steps: classical swine fever E2 truncated protein (TE2) coding gene is cloned into baculovirus vector pFastBacTM1, and is then transfected into Sf9 insect cells to obtain recombinant baculovirus capable of expressing protein TE2. The high five insect cells in logarithmic growth phase are infected by the recombinant baculovirus, so that a large amount of the protein TE2 can be expressed in a cell culture supernatant. Finally, the cell culture supernatant is recovered and purified to obtain a large amount of the recombinant protein TE2 with the purity more than 90%. According to the method, the target protein can be harvested from the cell culture supernatant, the time of protein purification is reduced, consumption of a large amount of time can be avoided, and the vaccine production process can be simplified. Under the premise of simplification of the vaccine production process, the recombinant protein TE2 has the advantages of strong immunogenicity and high safety, and the animal experiments prove that the recombinant protein can effectively stimulate the body to produce a highly effective humoral immune response.
Owner:NOVO BIOTECH CORP
RT-RPA (reverse transcription recombinase polymerase amplification) detection kit for fast detecting high-pathogenicity porcine reproductive and respiratory syndrome virus and application thereof
ActiveCN105567871AShorten test timeLow reaction temperatureMicrobiological testing/measurementMicroorganism based processesBiologyDifferential diagnosis
The invention discloses an RT-RPA (reverse transcription recombinase polymerase amplification) detection kit for fast detecting a high-pathogenicity porcine reproductive and respiratory syndrome virus and application thereof. The kit comprises a pair of primers and a probe, the sequences of the primers are shown as SEQ ID NO.1 and SEQ ID NO.2, and the sequence of the probe is shown as SEQ ID NO.3. It is proved through experiments that the kit can detect adverse effects of the high-pathogenicity porcine reproductive and respiratory syndrome virus (HP-PRRSV), a hog cholera virus, a C-type porcine reproductive and respiratory syndrome virus, a porcine circovirus type II, a porcine pseudorabies virus and a foot and mouth disease virus in a specificity mode. It is proved through experiments that the kit can detect out templates of at least 70 copies at the temperature of 40 DEG C on the condition of 20 min amplification, and the conformity between the kit and RT-qPCR is high. This shows that the kit can detect HP-PRRSV fast, efficiently and sensitively and provides an effective technological means for differential diagnosis of HP-PRRSV.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
RPA (recombinase polymerase amplification) primer and detection kit for rapidly detecting African swine fever viruses
PendingCN109797246AHigh sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesQuarantinePorcine reproductive and respiratory syndrome virus
The invention discloses an RPA (recombinase polymerase amplification) primer and a detection kit for rapidly detecting African swine fever viruses. Target genes can be effectively amplified, specificity is 100%, detection sensitivity is 102 copy / reaction, and sensitivity is equivalent to that of fluorescent quantitative PCR (polymerase chain reaction). Cross reaction between the RPA amplificationprimer and one of classical swine fever viruses, vesicular exanthema swine viruses I, porcine reproductive and respiratory syndrome viruses, porcine circoviruses and the like is omitted. A RPA isothermal amplification system is rapidness in reaction and wide in temperature range, effective amplification of the target genes can be achieved at the temperature of 38-46 DEG C, and the detection kit can rapidly, efficiently and sensitively detect the African swine fever viruses and has the advantages that the kit is simple to operate, high in specificity, safe and free from pollution, reaction results are easily observed and the like. Effective technical means are provided for on-site rapidness detecting and screening of infection nucleic acid of the African swine fever viruses, and the RPA amplification primer has great significance for control of infection spreading of the African swine fever viruses in China and inspection and quarantine in infected areas and entry and exit ports.
Owner:ACAD OF MILITARY SCI PLA CHINA ACAD OF MILITARY MEDICAL SCI INST OF MILITARY VETERINARY MEDICINE
Epitope antigen polypeptide of African swine fever virus and application of epitope antigen polypeptide of African swine fever virus
ActiveCN110618279AIncreased sensitivityStrong specificityBiological testingImmunoassaysEpitopeChemical synthesis
The invention discloses an epitope antigen polypeptide of an African swine fever virus and an application of the epitope antigen polypeptide of the African swine fever virus. The epitope antigen polypeptide of the African swine fever virus is a polypeptide as shown in a sequence 2 or a polypeptide as shown in a sequence 3 in a sequence table. A composition of the epitope antigen polypeptide is thecomposition formed by the polypeptide as shown in the sequence 2 in the sequence table and the polypeptide as shown in the sequence 3 in the sequence table. According to the epitope antigen polypeptide or the composition thereof disclosed by the invention, a detection kit is prepared by coating an elisa plate with a chemically synthesized antigen peptide, so that the epitope antigen polypeptide is small in amount of antigen, high in sensitivity and high in specificity; whether an African swine fever virus antibody exists or not can be efficiently detected; and the kit has the advantages of being high in sensitivity, good in specificity and convenient to operate, and has a good market prospect
Owner:CHINA ANIMAL HUSBANDRY IND
Classical swine fever virus E2 subunit vaccine and preparation thereof
The invention relates to a recombinant E2 protein by using silkworm as a platform to produce a classical swine fever virus gene subgroup 2.1. The invention also provides a subunit vaccine comprising the recombinant protein, which can be used for enhancing the capability of swine to resist classical swine fever virus infection.
Owner:黄金城
Test paper for identifying and detecting virulent strain and low virulent strain of hog cholera virus
The invention relates to livestock epidemic disease infection and immune identification and detection instruments, and in particular relates to a piece of test paper for identifying and detecting a virulent strain and a low virulent strain of hog cholera viruses. The test paper consists of a support plate, a sample pad, a gold mark pad, a detection membrane and a water absorbing pad, wherein a virulent infection detection line T1 '|', a low virulent infection detection line T2 '|' and a quality control line C '|' are arranged on the detection membrane. When the test paper is used, three red strips '|||' mean virulent virus infection of hog cholera viruses, two red strips '||' mean vaccine immunity of hog cholera viruses, and one red strip '|' means hog cholera viruse negative. The test paper is high in specificity, high in sensitivity, wide in reaction spectrum and simple, convenient and rapid to operate, can be used for detecting conventional low virulent vaccine strains and multiple epidemic virulent strains, can be widely used for identifying and detecting hog cholera virus infection and immune, and can be easily popularized and applied in production practice.
Owner:HENAN ACAD OF AGRI SCI
Hog cholera virus truncated E2 protein and application of same
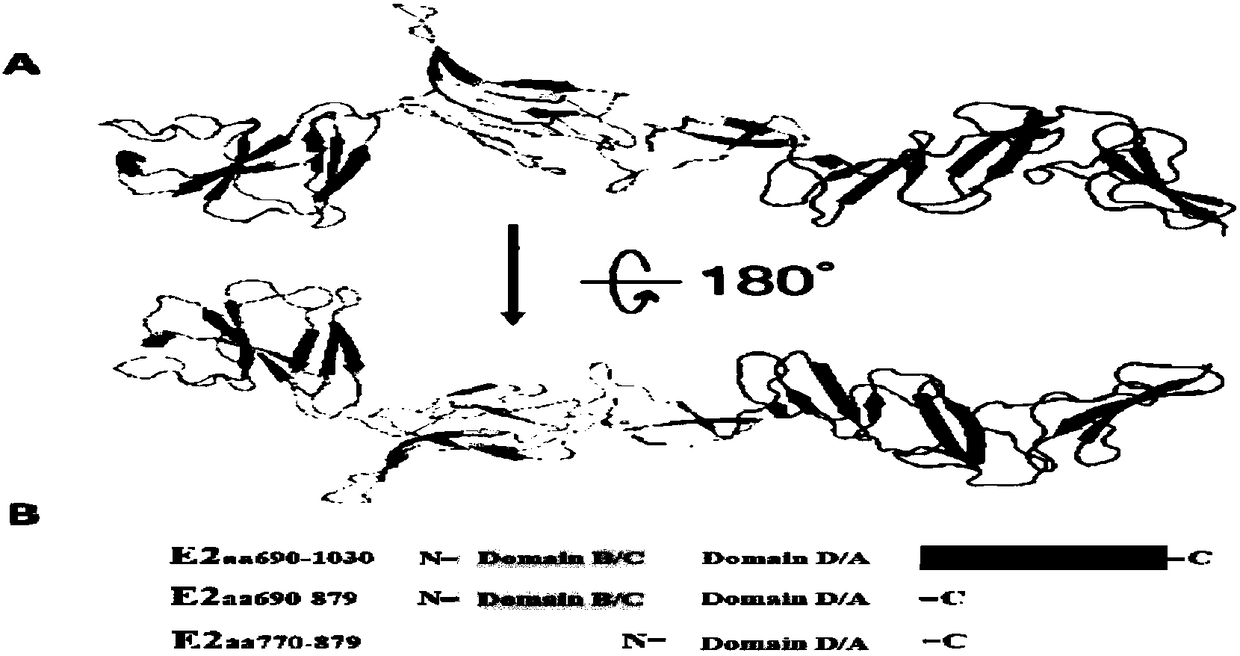
ActiveCN108107217APreserve antigenicityReduce manufacturing costBiological testingBovine Viral Diarrhea VirusesBiology
The invention discloses a hog cholera virus truncated E2 protein which is designed on the basis of protein spatial structure, and an application of the same. In the invention, according to the crystalstructure of bovine viral diarrhea virus E2 protein, the spatial structure of the hog cholera virus E2 protein is simulated, and then the hog cholera virus E2 protein is subjected to truncated expression, wherein the amino acid sequence of the truncated protein E2B / C / D / A is represented as the SEQ ID No.1. The truncated protein can maintain the complete antigenicity of the E2 protein, and has no cross reaction with a bovine viral diarrhea virus antibody. The invention further constructs a CHO cell line which stably expresses the truncated protein E2B / C / D / A and is assigned the accession numberof CGMCC No.14722. The invention also discloses an indirect ELISA kit which is used for detection of a hog cholera virus antibody, wherein the enveloped antigen is the hog cholera virus truncated protein E2B / C / D / A. The kit is used for specifically detecting the hog cholera virus antibody with high specificity, sensitivity and repeatability.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
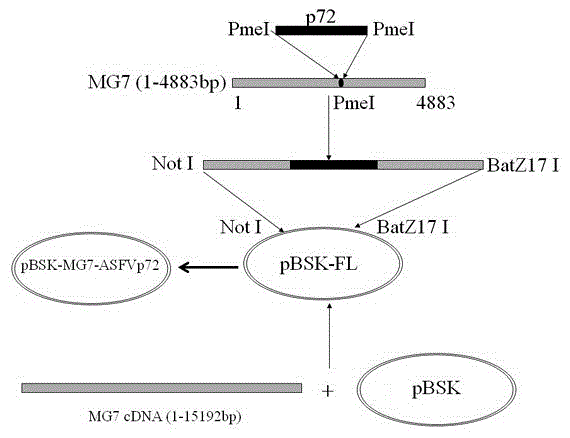
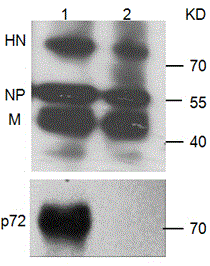
Recombined new castle disease virus vaccine strain for expressing African swine fever virus p72 proteins
ActiveCN104962581AImprove protectionEffective protectionViral antigen ingredientsMicroorganism based processesDiseaseNewcastle disease virus NDV
The invention provides a preparation method of a recombined new castle disease virus vaccine strain which expresses African swine fever virus p72 proteins and a recombined new castle disease virus vaccine strain. The method provided by the invention comprises the following steps: constructing a recombinant transcriptional plasmid which is inserted with a p72 gene of African swine fever virus (ASFV); constructing a transcriptional helper plasmid system; carrying out a contransfection for the transcriptional plasmids and the transcriptional helper plasmids into host cells BHK-21 which can be duplicated in new castle disease attenuate strains; and saving and obtaining the recombined virus stain. The vaccine strain for expressing the African swine fever virus p72 proteins provided by the invention has important preservation and strategy meaning in the aspect of animal epidemic disease prevention and control, and can be applied to the treatment and prevention of African swine fever virus.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Classical swine fever virus virulence determinant and a novel classical swine fever vaccine
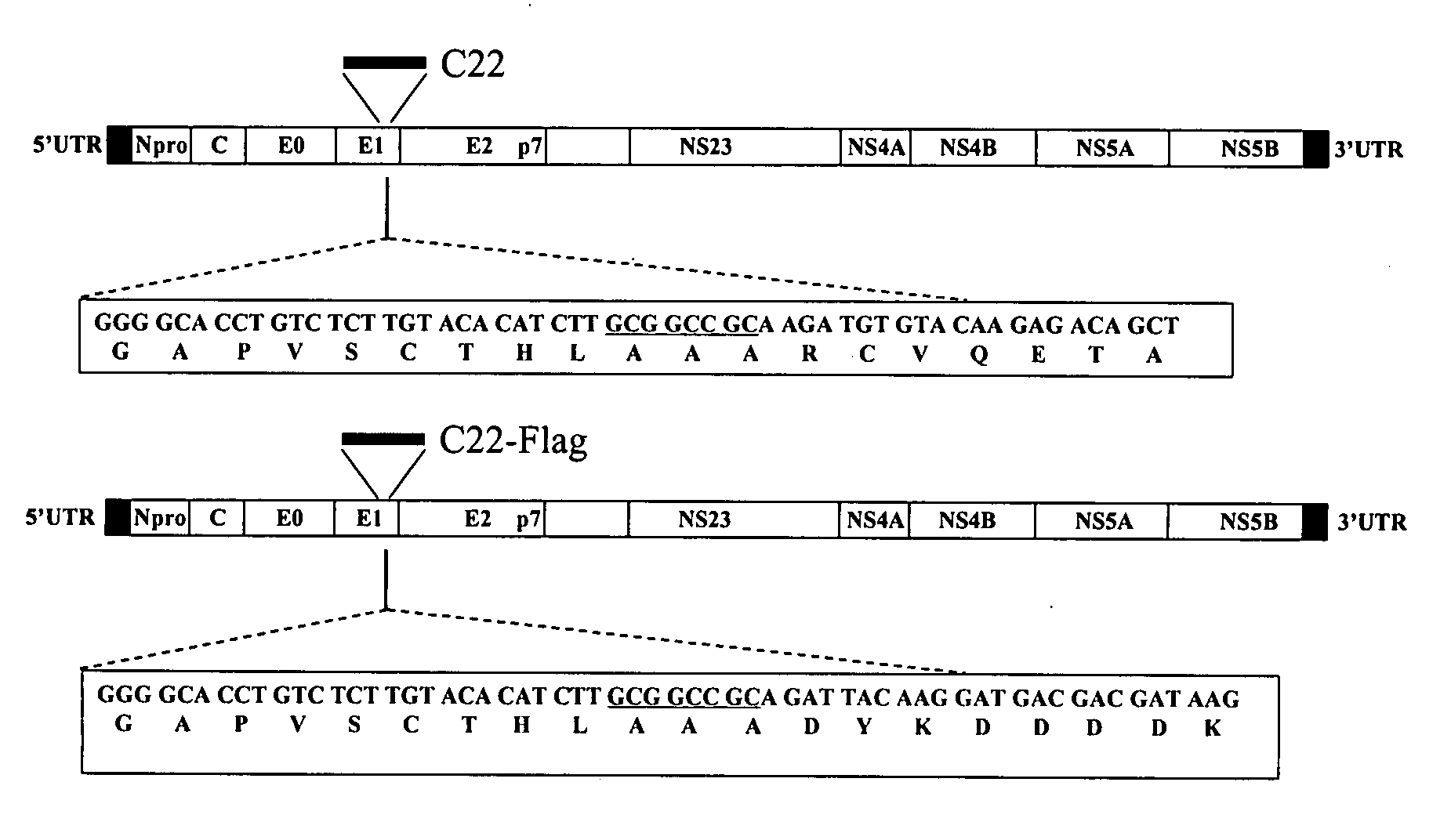
InactiveUS7332170B1Readily apparentSsRNA viruses positive-senseViral antigen ingredientsHighly pathogenicVirulent characteristics
Transposon linker insertion mutagenesis of a full-length infectious clone of the highly pathogenic classical swine fever virus (CSFV) isolate Brescia (pBIC) was used to identify genetic determinants of CSFV virulence and host range. A virus mutant, RB-C22 (RB-C22v), possessing a 19-residue tag insertion at the carboxyl end of E1 was constructed. RB-C22v and the parental virus pBIC (pBICv) exhibited similar growth characteristics on primary porcine macrophage cell cultures although RB-C22v produced significantly smaller plaques on SK6 cell cultures. In vivo, RB-C22v was markedly attenuated in swine. In contrast with pBIC infection, where mortality was 100%, all RB-C22v-infected pigs survived infection remaining clinically normal. Additionally, chimeras of the Brescia strain and the attenuated vaccine strain CS were constructed and evaluated for viral virulence in swine. Chimeras 138.8v and 337.14v, chimeras containing the E2 glycoprotein of CS and chimeric virus 319.1v, which contained only the CS E2 glycoprotein in the Brescia background, were attenuated in swine. Chimeras encoding all Brescia structural proteins in a CS genetic background remained attenuated, indicating that additional mutations outside the structural region are important for CS vaccine virus attenuation. The combined results indicate a significant role for E1 glycoprotein and E2 glycoprotein in swine virulence.
Owner:UNITED STATES OF AMERICA AS RESPRESENTED BY THE SEC OF AGRI THE
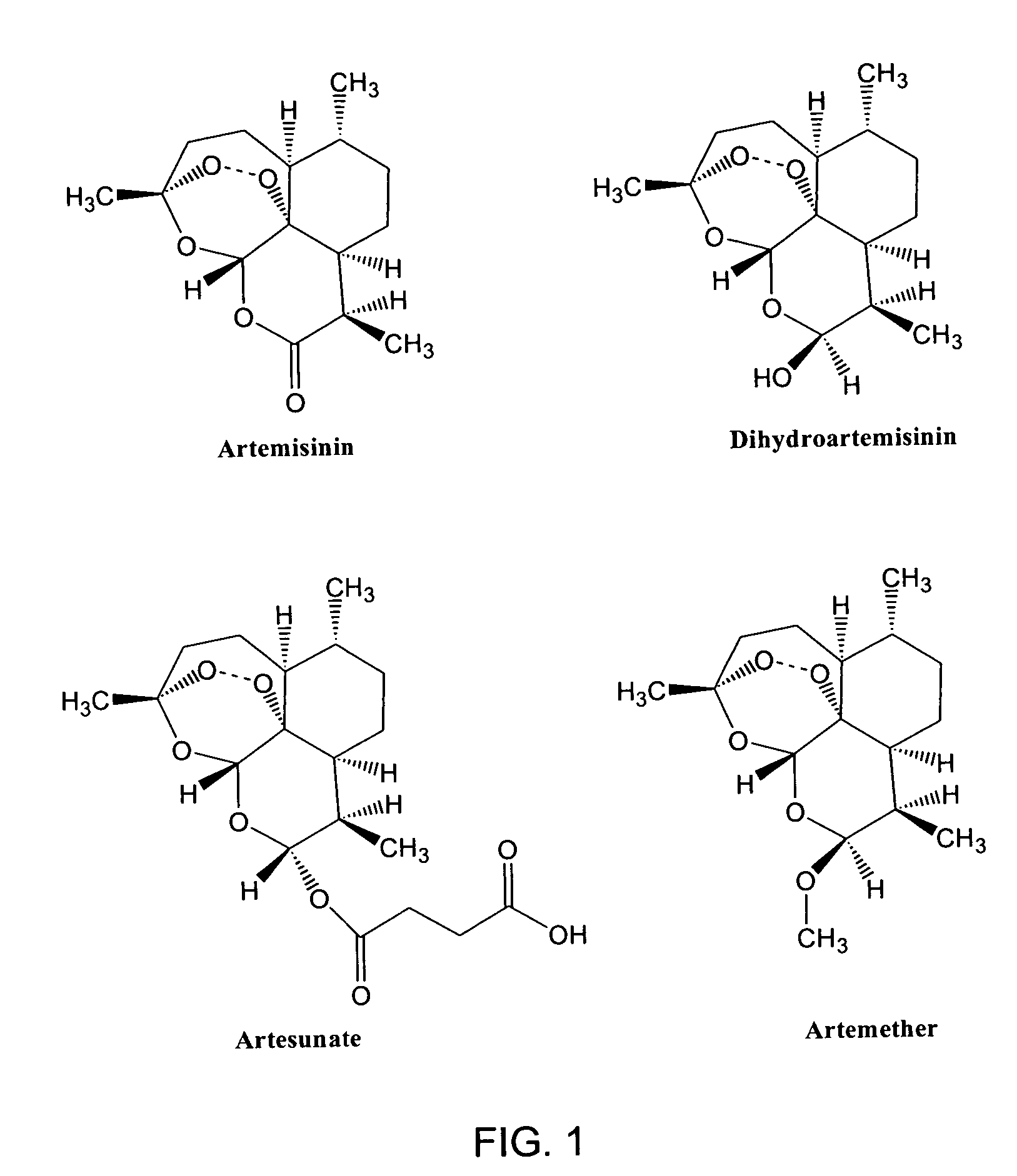
Use of endoperoxides for the treatment of infections caused by flaviviridae, including hepatitis C, bovine viral diarrhea and classical swine fever virus
InactiveUS20050059647A1Useful in treatmentBiocidePeptide/protein ingredientsAbnormal tissue growthBovine Viral Diarrhea Viruses
The use of sesquiterpenes and, in particular sesquiterpene lactone endoperoxides, such as artemisinin and analogs thereof, for the treatment of hepatitis C virus infections. Artemisinin, analogs of artemsisnin and some crude Artemisia extracts were tested in vitro against DNA-viruses, retro-viruses and Flavivirida, (an important family of human and animal RNA pathogens). These compounds were also screened for anti-tumor activity. Strong activity of artemisinin was noticed against the bovine viral diarrhea virus (BVDV). As pestiviruses, such as BVDV, share many similarities with hepatitis C virus (HCV), we can conclude that endoperoxides in general and artemisinin more specificly have efficacy as treatments for hepatitis C viral infections.
Owner:KEMIN FOODS L C
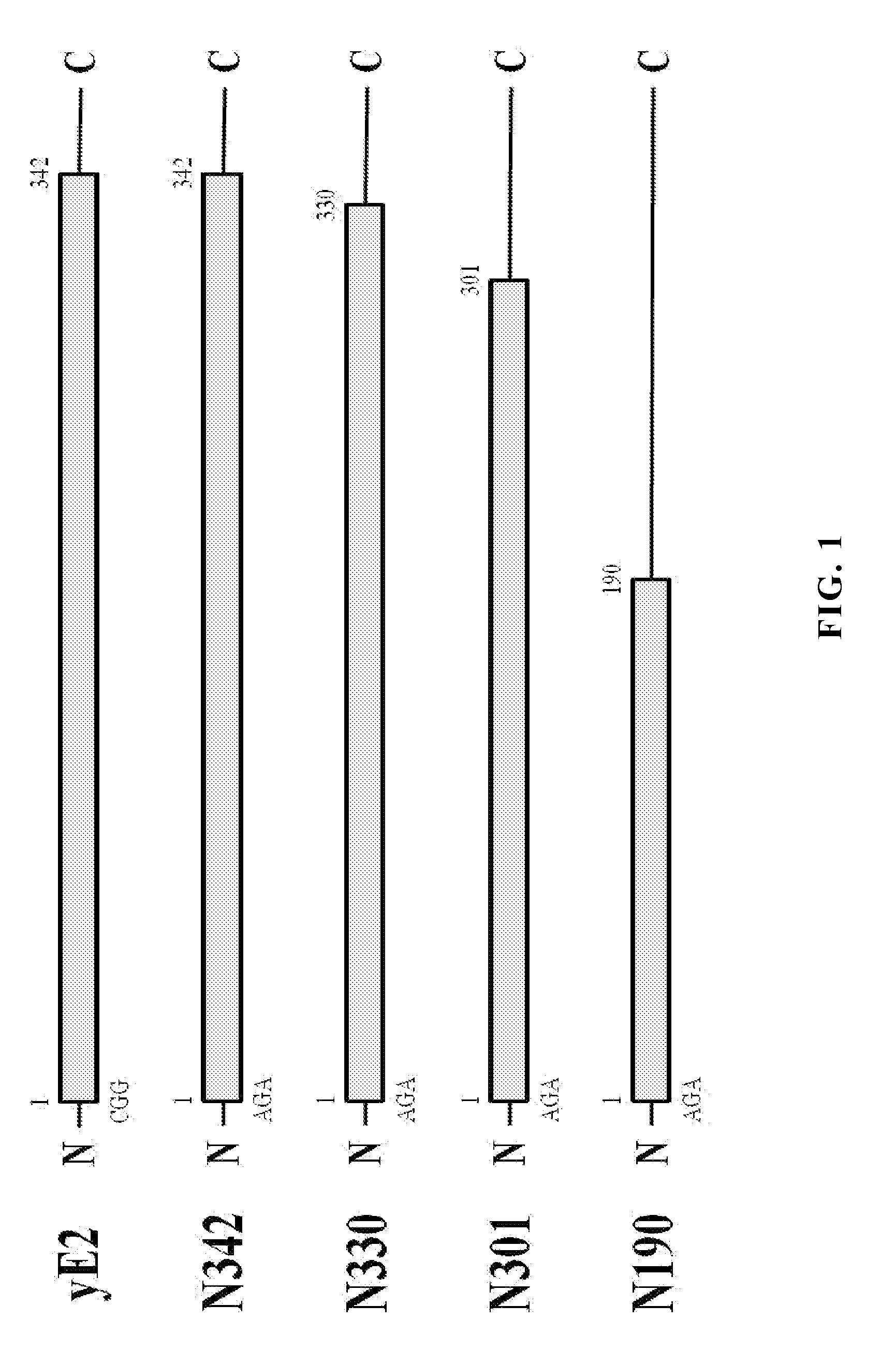
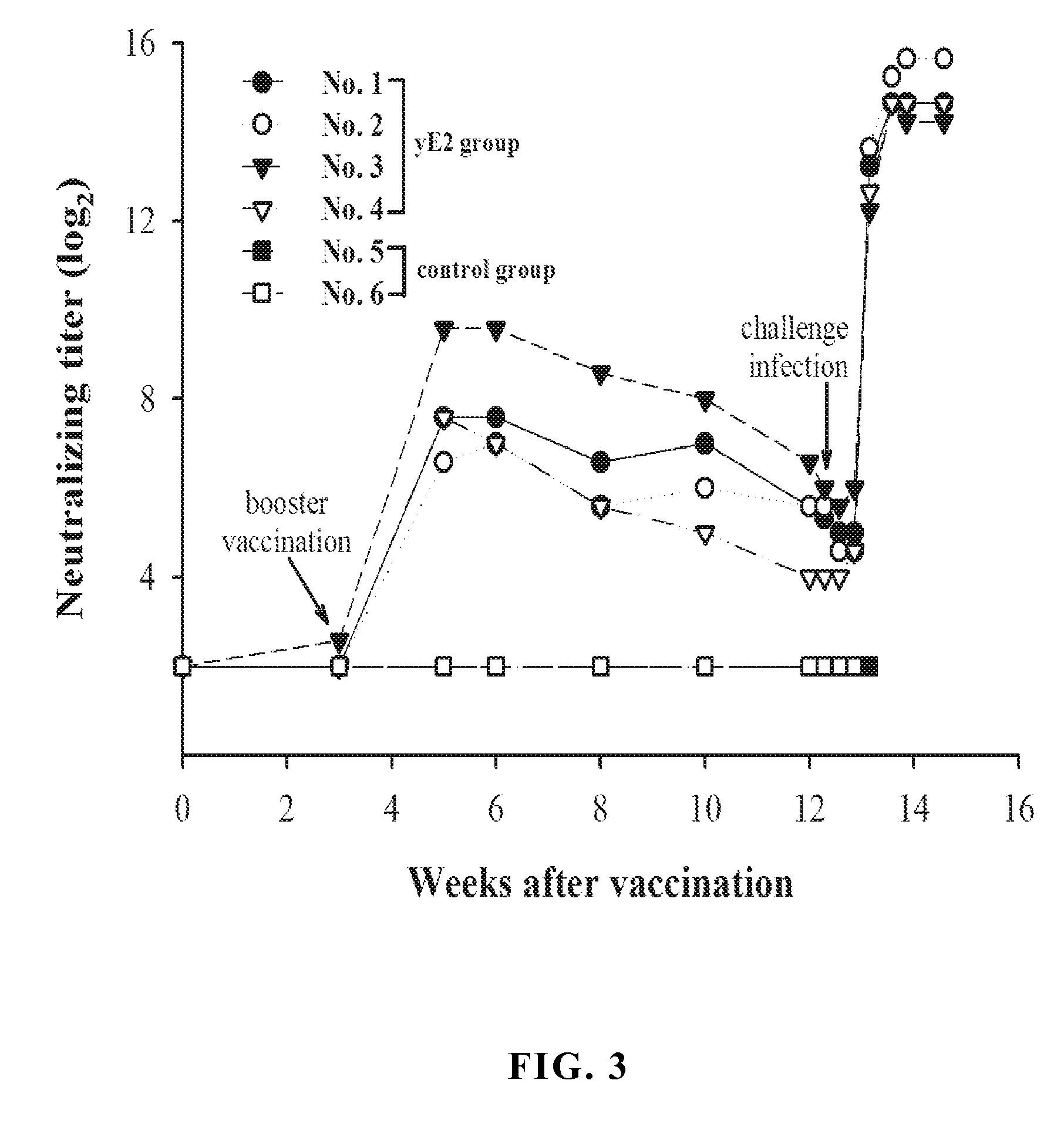
Yeast expressed classical swine fever virus glycoprotein e2 and use thereof
ActiveUS20140099338A1Improve the level ofHigh yieldSsRNA viruses positive-senseViral antigen ingredientsYeastElisa kit
The present invention provides a recombinant yeast system for expressing the glycoprotein E2 of classical swine fever virus (CSFV), in which the expression level of yE2 is improved by codon optimization and shortening coding region of E2 gene. The truncated E2 subunits are used as major active ingredient in anti-CSFV vaccines and useful diagnostic blocking ELISA kits for CSFV infection with easy manipulation and low cost.
Owner:MAO XING BIOLOGICAL TECH
Live attenuated antigenically marked classical swine fever virus
ActiveUS20080292653A1Effective protectionSsRNA viruses positive-senseAntibody mimetics/scaffoldsEpitopeStructural glycoprotein
Classical swine fever virus is a world-wide distributed highly-contagious disease affecting swine. The two main strategies for diseases control are prophylactic vaccination and non-vaccination stamping out policies. Marker vaccines are a promising strategy. Here we report the rational development of a doubly antigenic marker CSFV experimental live attenuated candidate strain vaccine (Flag / T4 virus). Flag / T virus (Flag / T4v) is based in the combination of two Brescia derived recombinant attenuated viruses: RB-C22 and T4. RB-C22v contains a 19mer insertion in the structural glycoprotein E1, while T4v posses mutated CSFV amino acid residues 830 to 834 in the structural glycoprotein E2, deleting the highly conserved epitope recognized by monoclonal antibody (mAb) WH303. Flag / T4 virus contains a positive foreign antigenic marker, due to the insertion of the highly antigenic epitope Flag in the 19mer insertion of E1, as well as a negative antigenic marker, the lack of reactivity with mAb WH303. Immunized with Flag / T4v induced a complete protection against the challenge with virulent strain Brescia both at 3 and 28 days post infection when nasally administered and since the second day post infection when intramuscularly administered. These results constitute an example of rational design of a CSFV antigenically marked LAV.
Owner:AGRI UNITED STATES OF AMERICA AS RESPRESENTED BY THE SEC OF THE +1
Method for preparing swine fever-pseudorabies bigeminal live vaccine and product thereof
InactiveCN101690808AImprove securityImprove immune efficiencyMicroorganism based processesAntiviralsImmunologic paralysisBiologic Products
The invention belongs to the technical field of biological products for veterinary use, in particular to a method for preparing a swine fever-pseudorabies bigeminal live vaccine by using passage cells and a product thereof. The method for preparing the swine fever-pseudorabies bigeminal live vaccine by using the passage cells comprises the following steps: (1) culturing a swine fever lentogen strain and a pseudorabies lentogen strain by the passage cells; (2) harvesting cell culture venoms prepared in the step (1); and (3) mixing two kinds of cell culture venoms prepared in the step (2) in a ratio of 1:2 to prepare the passage cell swine fever-pseudorabies bigeminal live vaccine through cooling and vacuum drying. The invention also provides the swine fever-pseudorabies bigeminal live vaccine prepared by the preparation method. The swine fever-pseudorabies bigeminal live vaccine can be used for immunization, can reduce the workload of the immunization, reduce the number of immunization times, avoid immunologic paralysis caused by frequent immunizations, reduce stress on a swine herd correspondingly, and prevent and control the occurrence of the swine fever and the pseudorabies.
Owner:广东永顺生物制药股份有限公司 +1
Anti-African swine fever antibody and preparation method thereof
PendingCN110078819AAchieve preparationAvoid destructionEgg immunoglobulinsImmunoglobulins against virusesYolkPig farms
The invention relates to the technical field of livestock immunology and antibody preparation and especially relates to an anti-African swine fever antibody and a preparation method thereof. The antibody is prepared according to the following steps: using an African swine fever virus for immunizing a healthy female poultry animal; collecting yolks after the content of African swine fever antibodyin the yolks of the immunized poultry animal exceeds 40ng / mL; extracting water-soluble components in the yolks; purifying, thereby acquiring the antibody. The preparation technology for producing theantibody is simple, low-cost and high-feasibility and can be used for large-scale preparation of African swine fever antibody. The acquired antibody can be applied to contaminated pig farms and earlyinfected swine, so as to reduce the duplication and propagation of African swine fever virus.
Owner:刘会芳
Recombinant BHK cell line for stably expressing classical swine fever virus E0-E1-E2 protein, and applications of the same in preparation of vaccines and diagnosis reagents of classical swine fever
ActiveCN103751773AFully emulsifiedAvoid infectionAntiviralsBiological testingStructural proteinMicrobiological culture
The present invention discloses a recombinant cell line for stably expressing classical swine fever virus E0-E1-E2 protein, and applications of the recombinant cell line in preparation of vaccines and diagnosis reagents of classical swine fever, wherein the recombinant cell line is BCSFV-E012, is preserved in the China General Microbiological Culture Collection Center, and has the preservation number of CGMCC No.7720. In addition, the present invention further discloses an establishment method for the cell line for stably expressing classical swine fever virus E0-E1-E2 protein, and a method for preparing a classical swine fever prevention vaccine composition by using the cell line. The present invention further discloses applications of the E0-E1-E2 protein stably expressed by the recombinant cell line in preparation of classical swine fever prevention vaccines and diagnosis reagents. The classical swine fever vaccine prepared by using the recombinant cell line has characteristics of high safety, good immunization effect, easy mass production, less being susceptible to exogenous virus pollution or influence of antibodies, and no classical swine fever virus non-structural protein antibody production so as to identify the vaccinated animal and the virus infected animal.
Owner:HARBIN WEIKE BIOTECH DEV +1
Use of hemagglutinin of the african swine fever virus as an adjuvant
InactiveUS20100086556A1Enhance immune responseHigh homologyOrganic active ingredientsVirusesHemagglutininAdjuvant
The invention generally relates to the use of the hemagglutinin (HA) of African swine fever virus (ASFV) as an adjuvant to enhance the immune response against an antigen in a subject. The invention provides a gene construct comprising all or part of the encoding sequence of said HA fused to the encoding sequence of an antigen. The invention is applicable in human and animal health.
Owner:FUNDACIO CENT DE RECERCA & SANITAT ANIMAL
Reagent kit special for testing high pathogenicity pig replication and syndrome virus variation strain
InactiveCN101220397AStrong specificityIncreased sensitivityMicrobiological testing/measurementHighly pathogenicPcr method
The invention provides a special kit for detecting highly pathogenic porcine reproductive and syndrome virus mutation strain. The highly pathogenic porcine reproductive and syndrome virus mutation strain is detected by self-designing specific primers PSX1 / PSX2, extracting virus RNA and carrying out reverse transcription into cDNA and utilizing a PCR method; the size of the estimated amplification segment of an amplification mutation strain is 404bp and an amplification traditional PRRSV amplification segment is 494bp. Compared with the prior art, the special kit has strong specificity and sensitivity, and the coincidence rate can achieve more than 90 percent compared with a virus separation and IPMA method. The amplification results of the primers to hogcholera virus, pseudorabies virus and porcine parvovirus are negative, which can distinguish a NSP2 mutation strain and a traditional strain; the method can detect the PRRSV with 12.8ng / L, thus the invention can be widely used for the clinical detection of the highly pathogenic porcine reproductive and syndrome virus mutation strain.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Yeast expressed classical swine fever virus glycoprotein e2 and use thereof
InactiveUS20100028384A1Induce productionProtection against CSFV infectionSsRNA viruses positive-senseViral antigen ingredientsEnationBULK ACTIVE INGREDIENT
A glycoprotein E2 of classical swine fever virus (CSFV) expressed in a recombinant yeast system. The recombinant E2 protein (yE2) is able to form a homodimer, exhibits glycosylation conformation and possesses correct immunogenicity. An anti-CSFV vaccine can be provided with yE2 as a major active ingredient to induce high titers of neutralizing antibody in vaccinated pigs, and to induce a protection against CSFV infection.
Owner:MAO XING BIOLOGICAL TECH
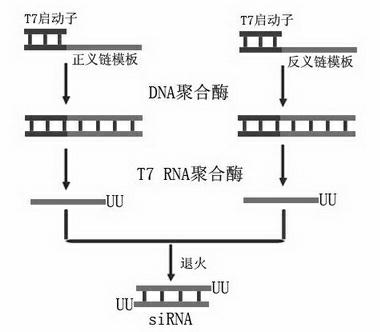
Small-interfering RNA (siRNA) capable of inhibiting classical swine fever virus (CSFV) reproduction and infection as well as preparation method and application thereof
InactiveCN102154293AAvoid infectionHigh suppression efficiencyGenetic material ingredientsAntiviralsViral replicationSmall interfering RNA
The invention discloses small-interfering RNA (siRNA) capable of inhibiting classical swine fever virus (CSFV) reproduction and infection as well as an encoding sequence thereof. The invention also discloses a method for preparing an anti-CSFV infection biological preparation using an RNA-interfering method. The siRNA is efficient and can specifically inhibit CSFV. The method is applicable to industrial production. The invention also provides a biological preparation produced by using the method. The biological preparation has the CSFV inhibition ratio of 95.04-98.59%, and can distinctly inhibit CSFV infection in sensitive animals, so as to prevent incidence and death of animals.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Novel recombinant baculovirus vector and uses thereof
ActiveUS20160068575A1Well formedSsRNA viruses positive-senseViral antigen ingredientsAntigenCircovirus
Disclosed herein is a recombinant viral construct and its uses thereof. The recombinant viral construct is capable of simultaneously expressing three exogenous proteins, including a classical swine fever virus (CSFV) antigen, a porcine circovirus type 2 (PCV2) antigen, and an immunomodulatory protein. The recombinant viral construct is hence useful as a bio-tool for simultaneously producing multiple antigens of a bi-subunit vaccine.
Owner:CHUNG YUAN CHRISTIAN UNIVERSITY
Vaccine composition, preparation method and application thereof
ActiveCN103908665AReduce the number of vaccinationsIncrease costAntibacterial agentsBacterial antigen ingredientsDiluentWater soluble
The invention relates to a vaccine composition, which contains classical swine fever virus antigen, porcine circovirus type 2 antigen, mycoplasma hyopneumoniae antigen and a vaccine adjuvant. The invention also provides a method for preparing the vaccine composition, a water-soluble vaccine adjuvant is used for preparing a mixture of the porcine circovirus type 2 antigen and mycoplasma hyopneumoniae antigen, and the mixture is taken as a diluent for diluting classical swine fever virus antigen. The vaccine composition has the advantages of simple preparation method, high titer content of the vaccine, and convenient and fast immunization, one time immunization can simultaneously preventing or treating swine fever, porcine circovirus type 2 antigen and mycoplasma hyopneumoniae, immunization cost is reduced, the immunization program is saved, and the application is more economic and reliable.
Owner:PU LIKE BIO ENG
Anti-African swine fever virus and anti-CD double-target pig-derived antibody, preparing method and application
PendingCN109734810ASolve the problem of mass deathAvoid damageBiocideHybrid immunoglobulinsAntigenInfected cell
The invention relates to the technical field of biological medicine, in particular to an anti-African swine fever virus and anti-CD double-target pig-derived antibody, a preparing method and application. The prepared anti-African swine fever virus and anti-CD pig-derived double-target compound antibody has two antigen combination sites which are combined with African swine fever viruses and immunecells respectively to generate the bridging effect and realize the two major functions; on one hand, the antigen proteins which cause immunosuppression and immune escape are inactivated, the immunosuppression and immune escape are eliminated, and the African swine fever viruses entering a body are killed; on the other hand, the functions of T cells are activated, the immune system is restored, the cell-mediated immunity is started, and virus expansion is prevented; meanwhile, infected cells are split and dissolved, phagocytes are activated to clear away the viruses and the infected cells, andthe aim of preventing and treating the African swine fever viruses is achieved. By means of the compound antibody, a biological medicine and a biological safety feed additive which eliminate the infection of the African swine fever viruses and prevent and treat the African swine fever can be prepared.
Owner:SHENZHEN JASON INTELLIGENT BIOTECH CO LIMLTED PRC
Preparation method of engineered vaccine based on CSF-FMD duplex gene
InactiveCN103933581APrevent and Control InfectionReduce economic lossGenetic material ingredientsAntiviralsGenetic engineeringAnimal body
The invention relates to a preparation method of an engineered vaccine based on a CSF-FMD duplex gene, and discloses research and application of a duplex nucleic acid vaccine based on hog cholera virus-O type hog foot-and-mouth disease virus. Main materials of the duplex nucleic acid vaccine based on hog cholera virus-O type hog foot-and-mouth disease virus are as follows: pcDNA3.1-CSFV-E2-FMDV-O-VP1 eukaryotic plasmids. The preparation method disclosed by the invention researches nucleic acid vaccines for CSFVE2 gene and the FMDV-OVP1, can stimulate an animal body to generate corresponding high-level antibody and show very strong specificity. For the hog infection and propagation caused by CSFV and FMDV-O, the E2 gene and the VP1 gene achieve prevention and control effect on two viruses, so that safe and effective effects can be achieved.
Owner:GUIZHOU UNIV
Recombinant baculovirus vector and uses thereof
Disclosed herein is a recombinant viral construct and its uses thereof. The recombinant viral construct is capable of simultaneously expressing three exogenous proteins, including a classical swine fever virus (CSFV) antigen, a porcine circovirus type 2 (PCV2) antigen, and an immunomodulatory protein. The recombinant viral construct is hence useful as a bio-tool for simultaneously producing multiple antigens of a bi-subunit vaccine.
Owner:CHUNG YUAN CHRISTIAN UNIVERSITY
Vaccine composition for classical swine fever from plant and manufacturing method thereof
ActiveUS20180305710A1Improve efficiencyImprove securitySsRNA viruses positive-senseViral antigen ingredientsCelluloseAntigen
A recombinant vector for transforming a plant, a plant transformed with the recombinant vector, a plant-made classical swine fever virus antigen pmE2 protein expressed in the plant and uses thereof is provided. By using a recombinant vector having a polynucleotide encoding a GP55 protein of CSFV according to the present invention; and a polynucleotide encoding a cellulose-binding domain protein; and a plant transformed with the recombinant vector, a plant-made classical swine fever virus antigen pmE2 protein may be produced with high efficiency, and has higher safety and stability than those achieved by other production methods. Also, since the plant-derived classical swine fever virus antigen protein pmE2 has a cellulose-binding domain (CBD) protein, it may be usefully used as an effective marker to determine a virus exposure pathway and an antibody producing pathway.
Owner:BIOAPPL +1
Dual real-time fluorescence RT-PCR (reverse transcription-polymerase chain reaction) kit for detecting and identifying wild strain and vaccine strain of CSFV (classical swine fever virus) in swine umbilical cord blood and application of dual real-time fluorescence RT-PCR kit
InactiveCN106435033ANo cross contaminationEfficient detectionMicrobiological testing/measurementBorder disease virusBovine Viral Diarrhea Viruses
The invention discloses a dual real-time fluorescence RT-PCR (reverse transcription-polymerase chain reaction) kit for detecting and identifying a wild strain and a vaccine strain of a CSFV (classical swine fever virus) in swine umbilical cord blood and an application of the dual real-time fluorescence RT-PCR kit. The kit comprises a pair of primers and two fluorescent probes, wherein the sequences of the pair of primers are shown as SEQ ID NO.1 and SEQ ID NO.2, and the sequences of the two fluorescent probes are shown as SEQ ID NO.3 and SEQ ID NO.4. The kit has the advantages of high specificity, sensitivity and accuracy and excellent repeatability, meanwhile, the wild strain and the vaccine strain of the CSFV can be identified and detected by detecting the same sample once, and the problem of genetic crossover of the CSFV with the bovine viral diarrhea virus and the border disease virus which belong to the same genus can be solved. The kit is applicable to fast identification and detection of the wild strain and the vaccine strain of the CSFV in scientific research and clinical detection, can be used for precisely evaluating and diagnosing CSFV carrying and expelling conditions of sows and latent infection conditions of piglets and can be further used for evaluating effects of CSFV vaccines.
Owner:HUNAN XINNANFANG CULTURE SERVICE CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com