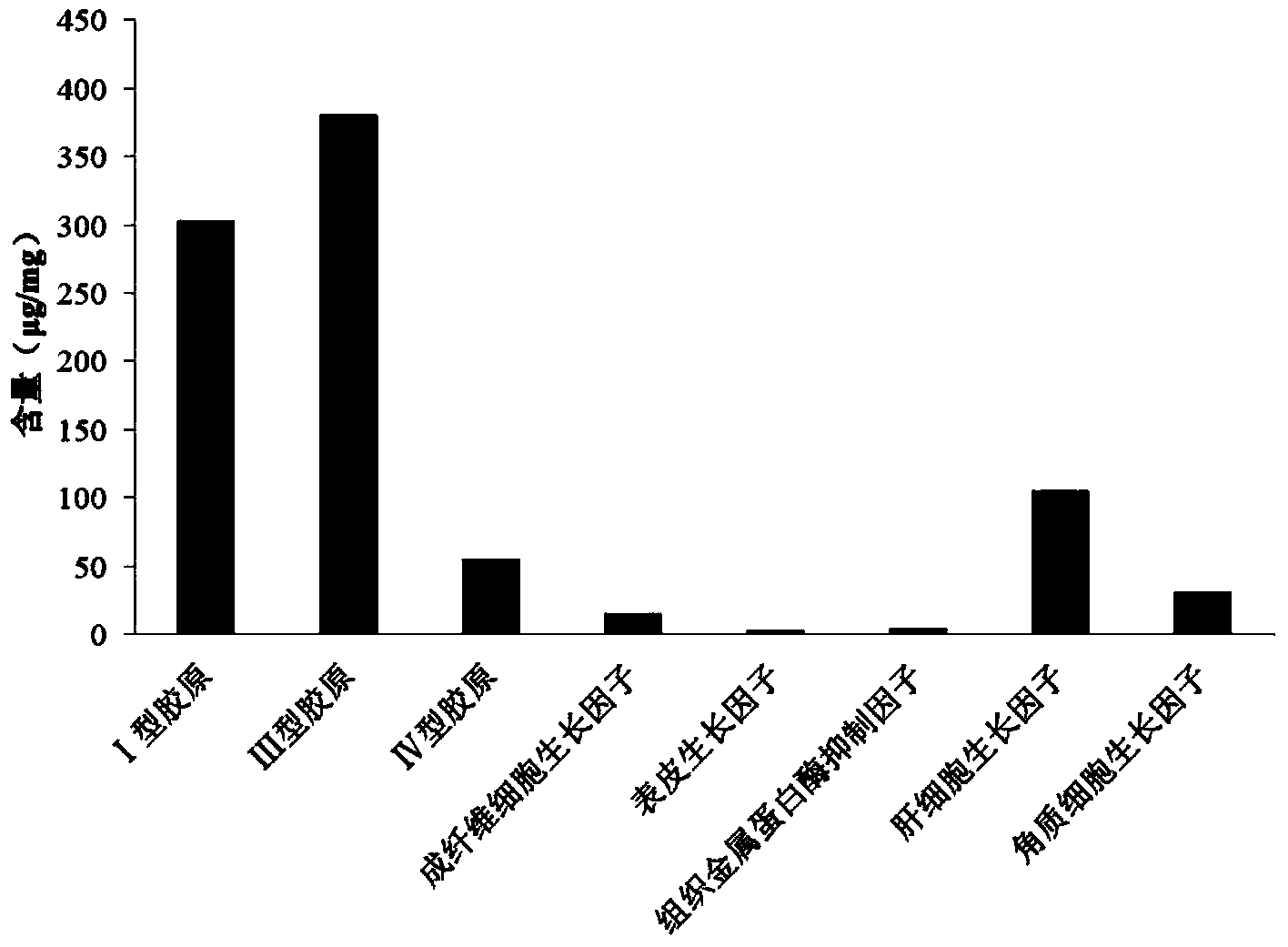Patents
Literature
176results about How to "High homology" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Control of gene expression
InactiveUS20030074684A1Homology of non-coding segments will be equally effectiveHigh homologyAntibacterial agentsFungiBiological bodySynthetic Genes
The present invention relates generally to synthetic genes for modifying endogenous gene expression in a cell, tissue or organ of a transgenic organism, in particular a transgenic animal or plant. More particularly, the present invention provides novel synthetic genes and genetic constructs which are capable of repressing delaying or otherwise reducing the expression of an endogenous gene or a target gene in an organism when introduced thereto.
Owner:BENITEC AUSTRALIA +1
Method to clone mRNAs
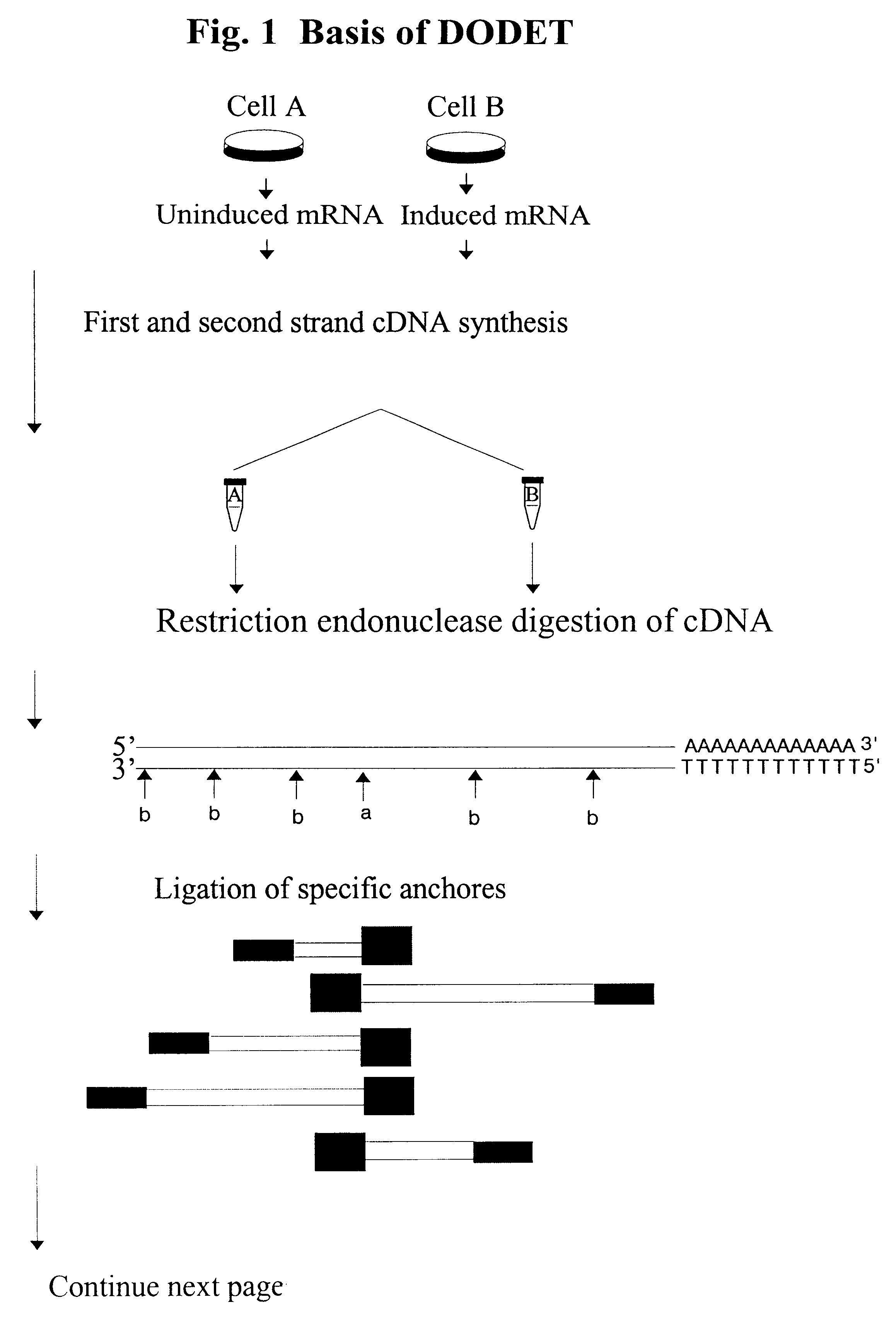
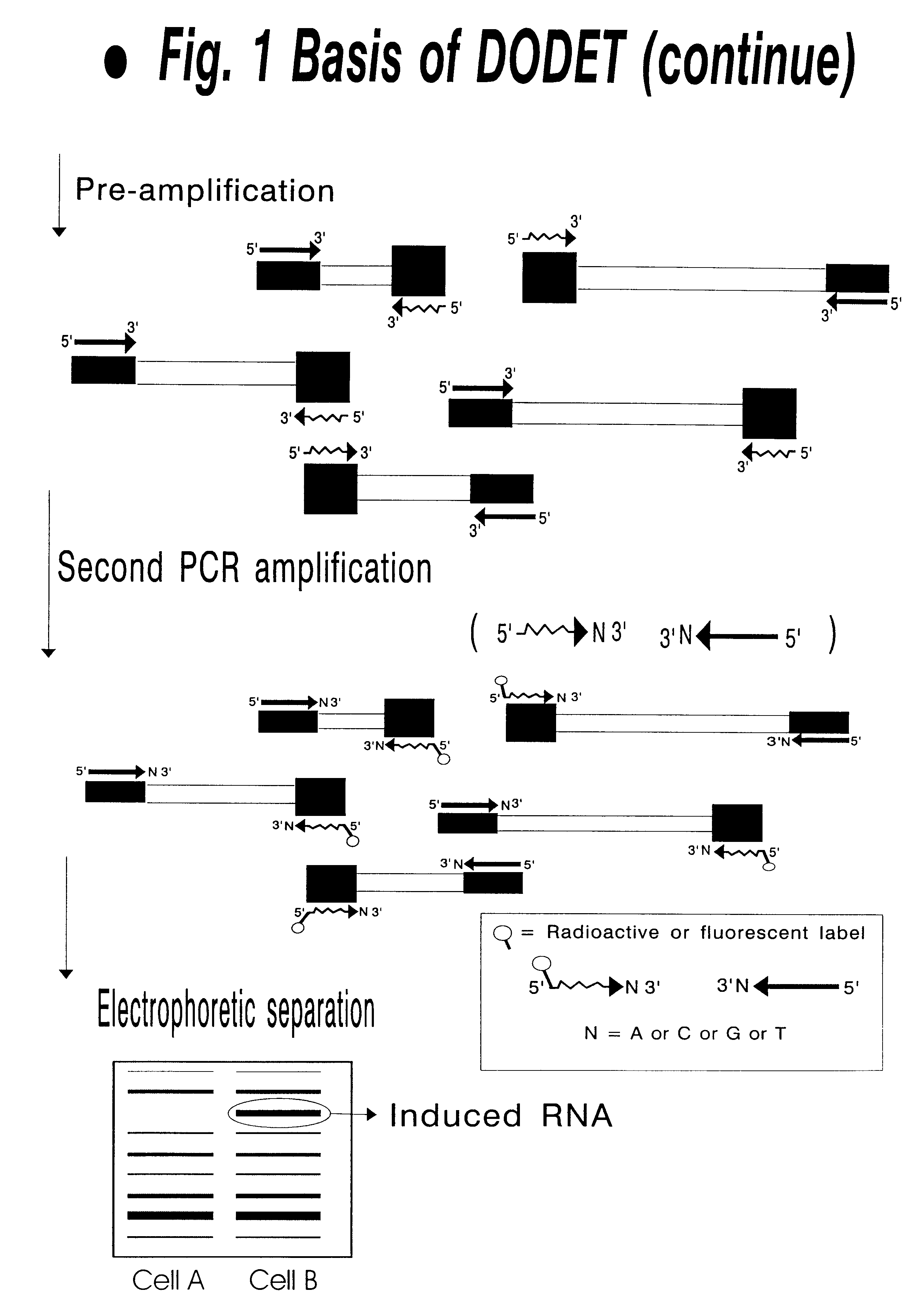
InactiveUS6261770B1Convenient verificationHigh homologySugar derivativesMicrobiological testing/measurementNucleotideNucleotide sequencing
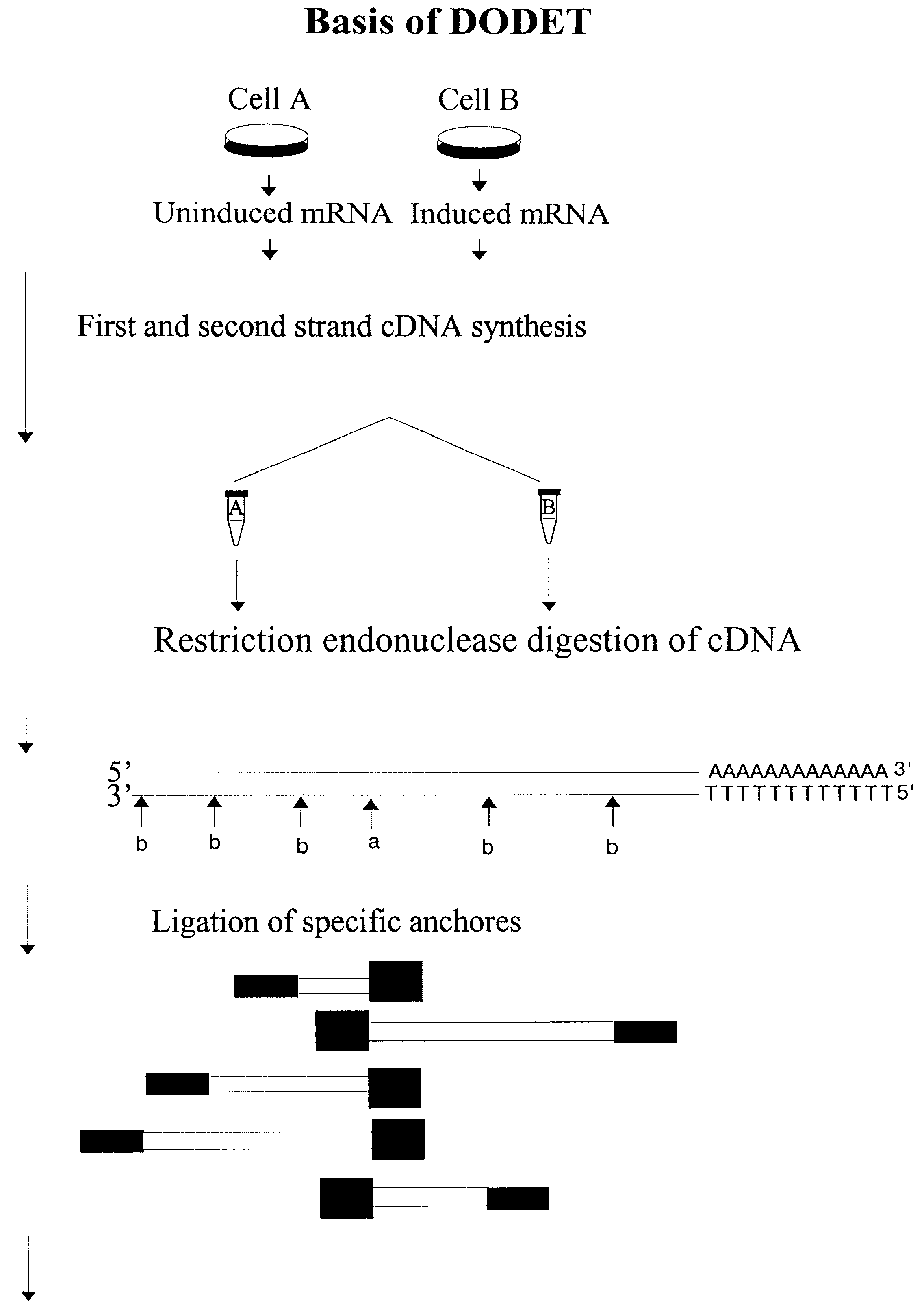
Disclosed and claimed is a method for preparing a normalized sub-divided library of amplified cDNA fragments from the coding region of mRNAs contained in a sample. The method includes the steps of: a) subjecting the mRNA population to reverse transcription using at least one cDNA primer, thereby obtaining first strand cDNA fragments, b) synthesizing second strand cDNA complementary to the first strand cDNA fragments by use of the first strand DNA fragments as templates, thereby obtaining double stranded cDNA fragments, c) digesting the double stranded cDNA fragments with at least one restriction endonuclease, the endonuclease leaving protruding sticky ends of similar size at the termini of the DNA after digestion, thereby obtaining cleaved cDNA fragments, d) adding at least two adapter fragments containing known sequences to the cleaved cDNA fragments obtained in step c), the at least two adapter fragments being able to bind specifically to the sticky ends of the double stranded cDNA produced in step c), the one adapter fragment being able to anneal to the primer having formula I in step f), the second adapter fragment being a termination fragment introducing a block against DNA polymerization in the 5'->3' direction setting out from said termination fragment and the termination fragment being unable to anneal to any primer of the at least two primer sets in step f) during the molecular amplification procedure, the at least two adapter fragments being ligated to the cleaved cDNA fragments obtained in step c) so as to obtain ligated cDNA fragments, e) sub-dividing the ligated cDNA fragments obtained in step d) into 4n1 pools where 1<=n1<=4, and f) subjecting each pool of ligated cDNA fragments obtained in step e) to a molecular amplification procedure so as to obtain amplified cDNA fragments, wherein is used, for an adapter fragment used in step d), a set of amplification primers having the general formula Iwherein Com is a sequence complementary to at least the 5'-end of an adapter fragment which is ligated to the 3'-end of a cleaved cDNA fragment, N is A, G, T, or C, the one primer having the general formula I where n1=0, and the second primer having the general formula I where 1<=n1<=4, the second primer being capable of priming amplification of any nucleotide sequence ligated in its 3'-end to the adapter fragment complementary in its 5'-end to Com.
Owner:AZIGN BIOSCI
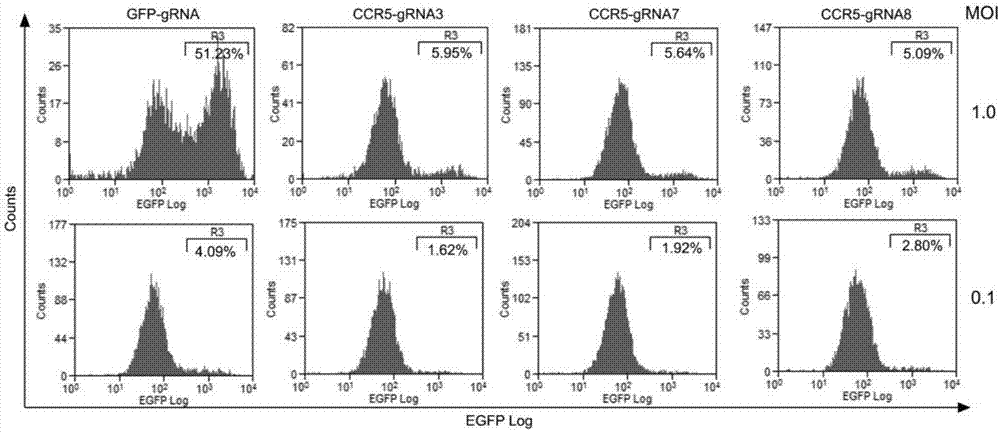
CRISPR (clustered regularly interspaced short palindromic repeat)/Cas9 Recombinant lentiviral vector containing gRNA sequence specifically targeting CCR5 and application thereof
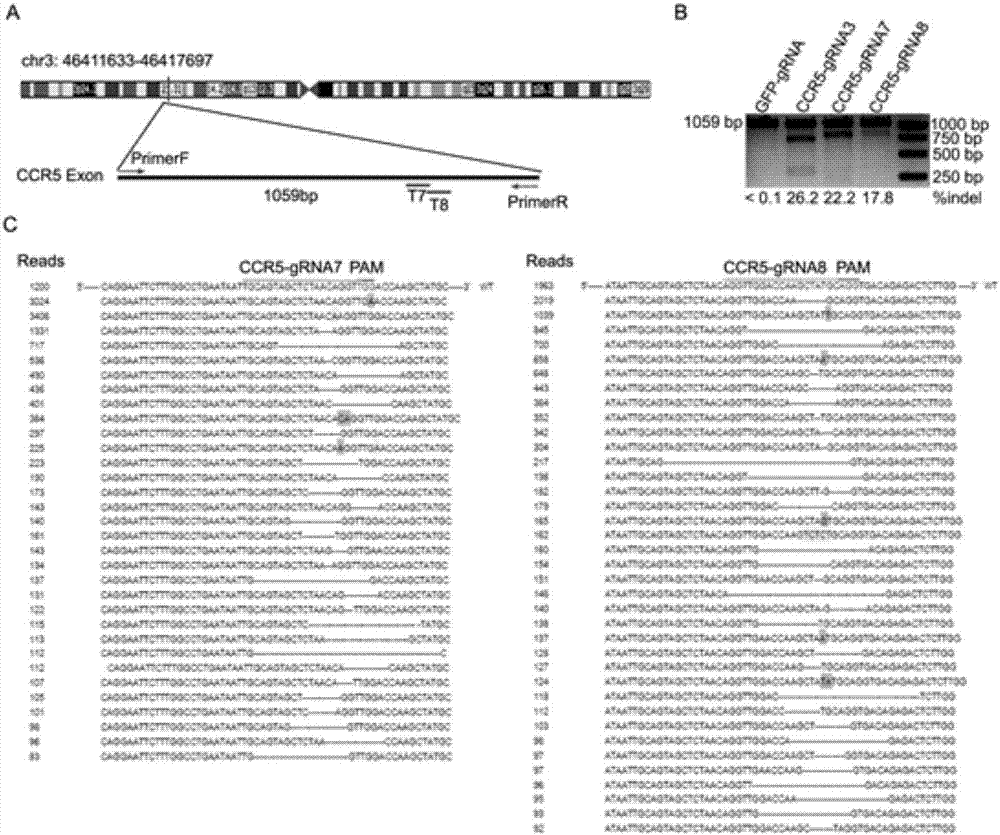
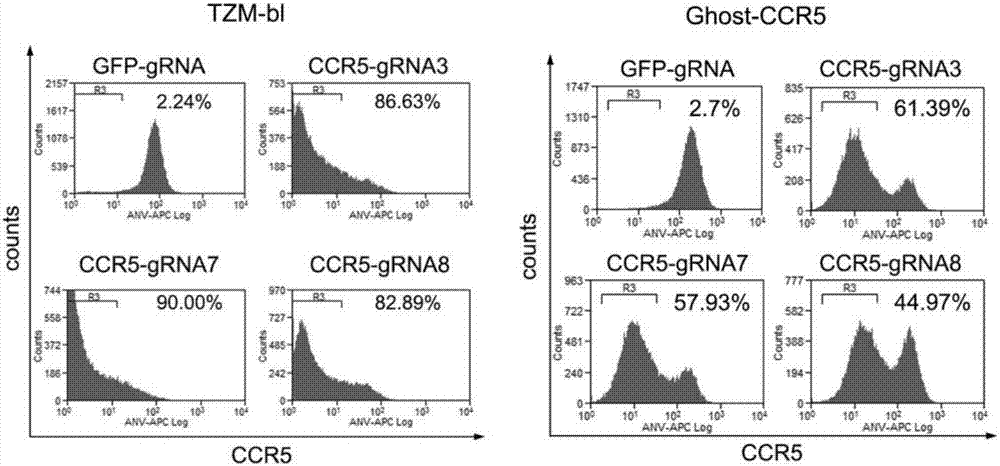
ActiveCN107312798AAvoid infectionInfection efficiency dropsOrganic active ingredientsGenetic material ingredientsRandom mutationLentivirus
The invention discloses a CRISPR (clustered regularly interspaced short palindromic repeat) / Cas9 recombinant lentiviral vector containing gRNA sequence specifically targeting CCR5 and application thereof. A lentivirus of the CRISPR / Cas9 recombinant lentiviral vector containing gRNA sequence specifically targeting CCR5 gene Delta 32 region is constructed that the lentivirus can introduce cells into a CRISPR / Cas9 system specific to CCR5, double-chain breakage occurs to a specific site of CCR5 gene, a random mutation is introduced to a breakage site after repairing by means of nonhomogeneous recombinant terminal binding, and the mutation rate reaches 90% and above. As gRNA is a nonhomogeneous region of CCR5 and CCR2, detection shows that the missing efficiency of the two gRNAs is lower than 0.2%. Cells modified via the recombinant lentivirus have significantly decreased efficiency of HIV (human immunodeficiency virus) infection. The system is quick to construct, simple and low in price, and is applicable to gene therapy of acquired immune deficiency syndrome.
Owner:WUHAN UNIV
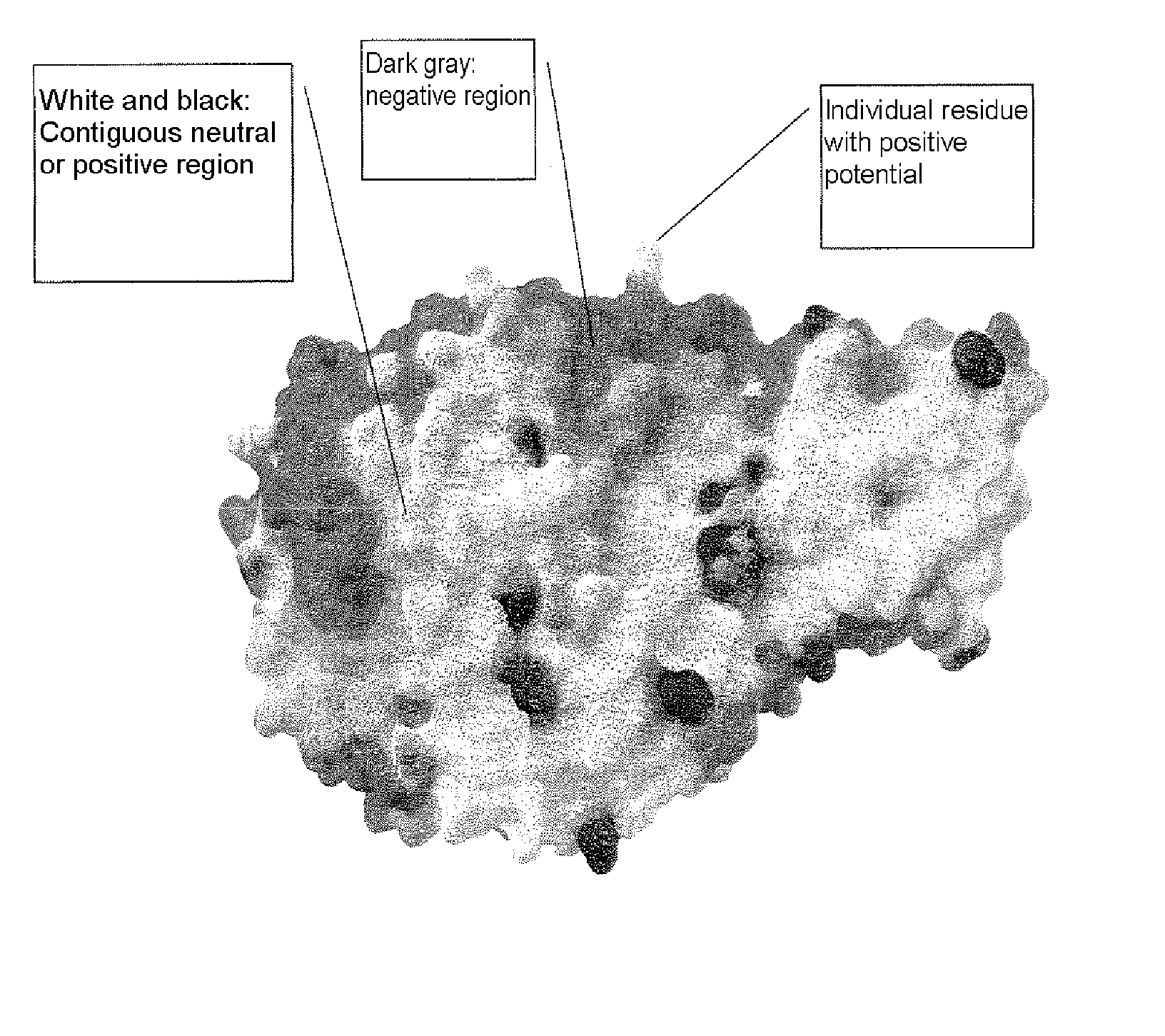
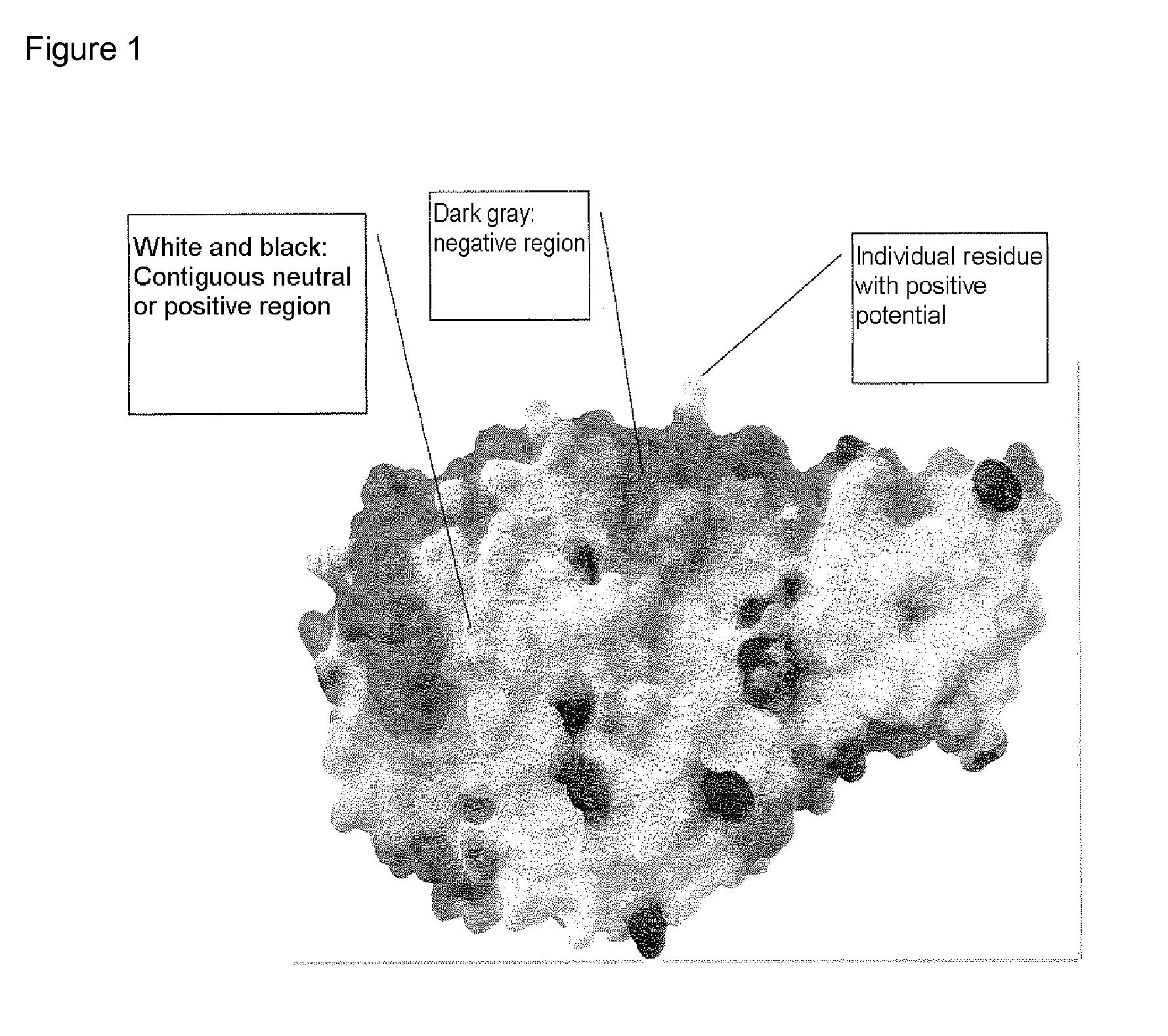
Alpha-amylase variants stabilized against dimerization and/or multimerization, method for the production thereof, and detergents and cleansers containing these alpha-amylase variants
InactiveUS20070212768A1Improve stabilityHigh homologyDetergent compounding agentsPancreatinAlpha-amylaseSolvent
The present invention relates to α-amylase variants that are stabilized to dimerization and / or multimerization, in particular at elevated temperatures or high pH, by point mutagenesis of positively polarized or charged or neutral surface amino acids to give more negatively polarized or charged amino acids. The invention further relates to methods of increasing the stability of an α-amylase to dimerization and / or multimerization brought about by electrostatic interactions whereby at least one amino acid residue on the surface of the starting molecule, which makes a neutral or positively polar or charged contribution to the electrostatic potential of said molecule, is replaced with a more negatively polar or negatively charged amino acid residue. The α-amylase variants obtained thereby exhibit better stability to influences of the solvent, increased processivity, and are suited for numerous industrial areas of use, in particular as active ingredients in detergents and cleansers.
Owner:BASF AG
Fish broad-spectrum vibrio subunit vaccine and preparation method
InactiveCN102512674AImprove resistanceImprove immunityAntibacterial agentsMicroorganism based processesFlagellinVibrio alginolyticus
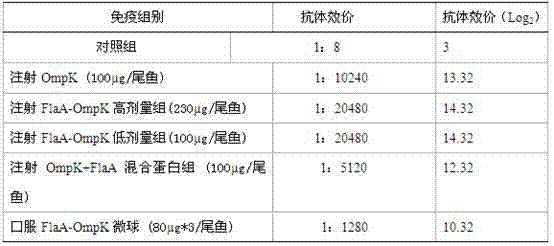
The invention discloses a broad-spectrum vibrio subunit vaccine for preventing fish pathogenic vibrio infection and a preparation method, belonging to the technical field of biology. The fusion protein OmpK-FlaA of an outer membrane protein OmpK with a conserved structure and a flagellin protein FlaA on the vibrio parahaemolyticus surface serves as an antigen component of the vaccine. The method is characterized by comprising the following steps of: superposing and extending Ppolymerase chain reaction (PCR) to the ompK and flaA genes of the vibrio parahaemolyticus to obtain a fusion protein gene flaA-ompK; constructing flaA-ompK-pET-28a recombinant plasmids; and obtaining the fusion FlaA-OmpK with high purity through exogenous induction expression and purification. The vaccine prepared bythe invention is safe and nontoxic and has no side effects; injection immune can be adopted; and the immune can also be realized by taking enteric microsphere vaccine orally; cross immunity protection can be acted to fish pathogenic vibrio (vibrio parahaemolyticus, vibrio alginolyticus, vibro harveyi, vibrio anguillarum and vibrio vulnificus).
Owner:FUZHOU UNIVERSITY
African swine fever fluorescent PCR (polymerase chain reaction) assay reagent, African swine fever fluorescent PCR assay kit and application thereof
ActiveCN106957927AIncreased sensitivityHigh homologyMicrobiological testing/measurementMicroorganism based processesFluorescenceAfrican swine fever
The invention relates to an African swine fever fluorescent PCR (polymerase chain reaction) assay reagent, an African swine fever fluorescent PCR assay kit and application thereof, which belong to the field of bioengineering. With the p72 gene as a reference, a pair of specific PCR primers and a TaqMan fluorescent probe are designed and optimized. Moreover, the primers are designed with the full length of the p72 gene to amplify the p72 gene, and the PCR product is cloned into a pGEM-T vector. Standard curves are drawn with a positive plasmid as a standard product, and the assay range is 10 to 1.0*10<7> copies. In the whole process of assaying African swine fever by an African swine fever fluorescent PCR method disclosed by the invention, only 2.5 to 3 hours are taken from DNA extraction to the appearance of an assay result, manual operation is greatly reduced, and time is greatly shortened. Furthermore, a plurality of samples can be assayed each time, and particularly assay of a large batch of samples can be realized.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Method, kit and oligonucleotide for detecting novel coronavirus
PendingCN111304368ASolve missing detectionAvoid Sampling MistakesMicrobiological testing/measurementMicroorganism based processesNucleotideGenome
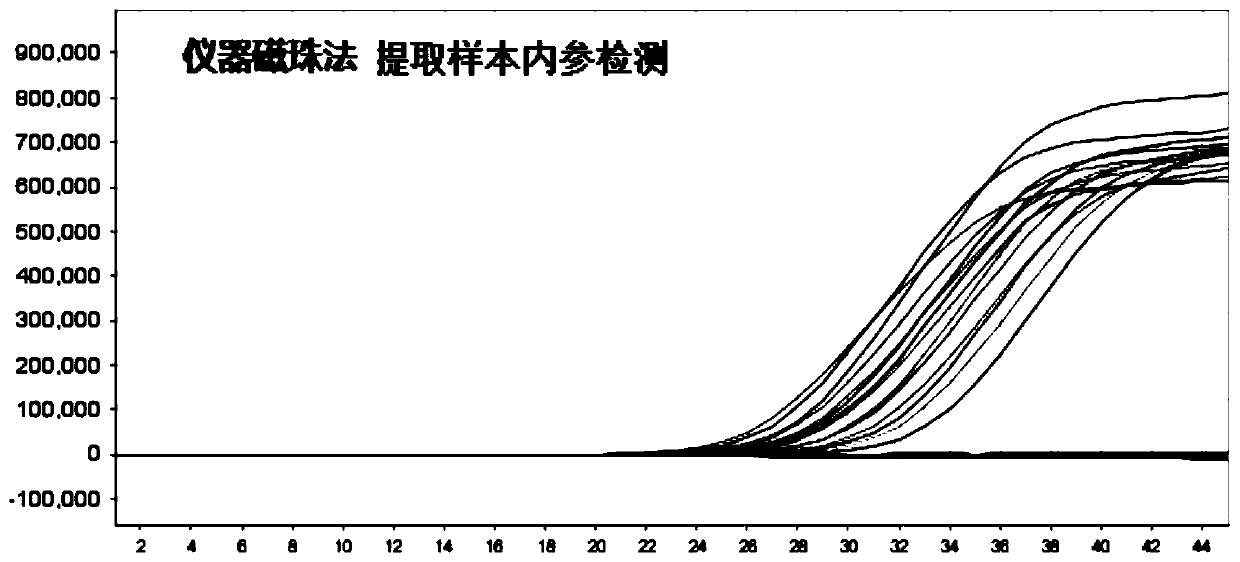
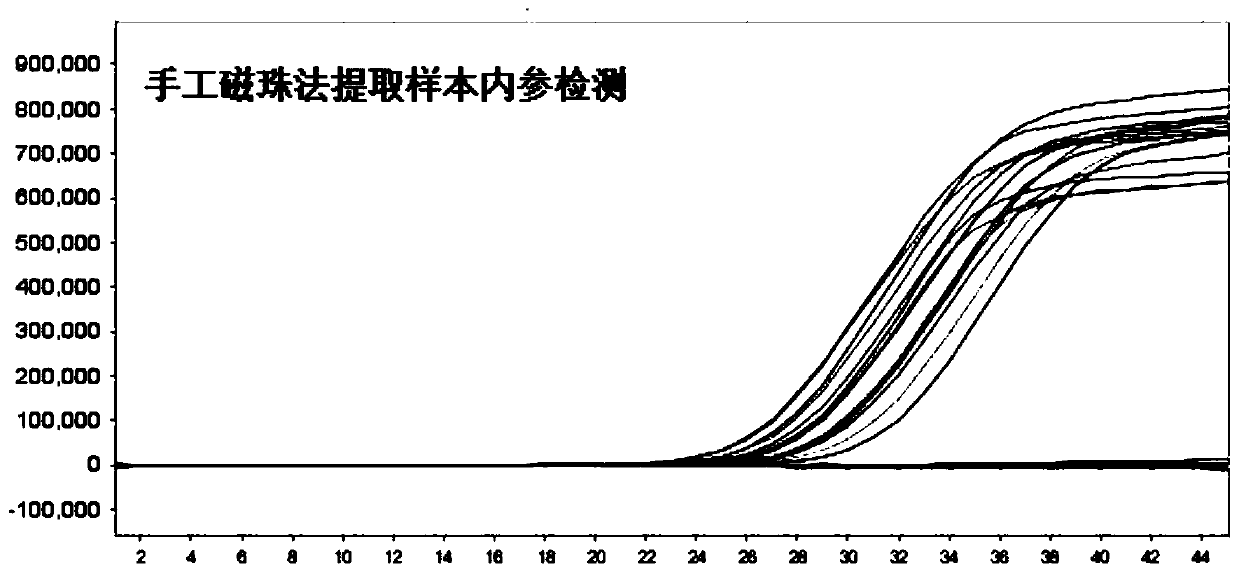
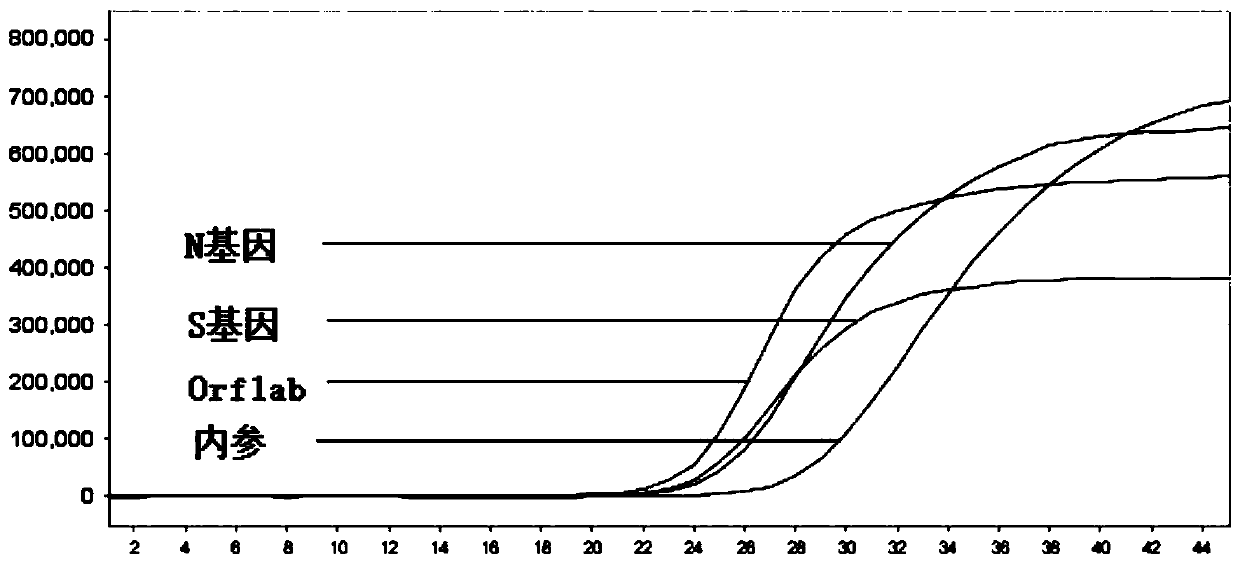
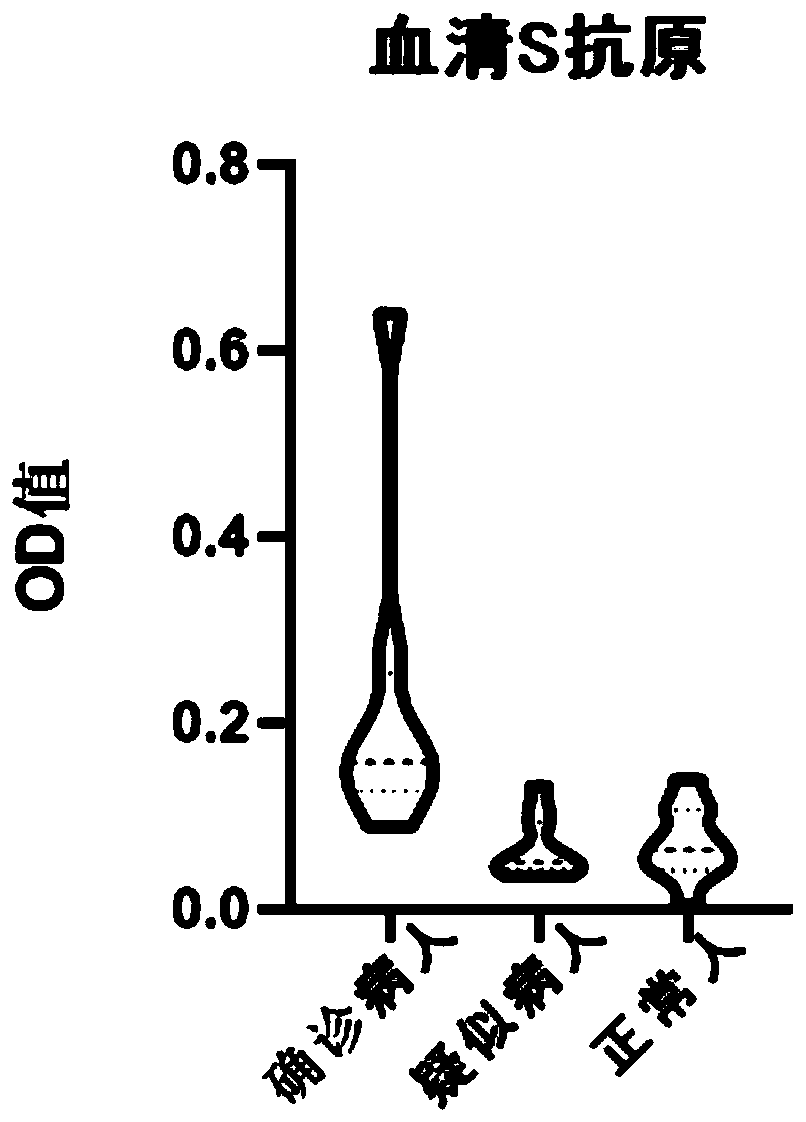
The invention discloses an oligonucleotide, a kit and a method for detecting novel coronavirus SARS-CoV-2, and relates to a primer and a probe for designing specificity aiming at Orf1ab, N and S generegions in a novel coronavirus genome. After nucleic acid in a sample is rapidly extracted, a one-step fluorescent RT-PCR technology is adopted to detect novel coronavirus nucleic acid. The method issimple to operate, and three target genes of the novel coronavirus are detected at the same time, so that missing detection can be avoided.
Owner:ACON BIOTECH (HANGZHOU) CO LTD
Cotton GhZFP1 gene sequence, its clone and use
InactiveCN1818065AImprove salt toleranceHigh homologyPlant peptidesFermentationCDNA libraryNicotiana tabacum
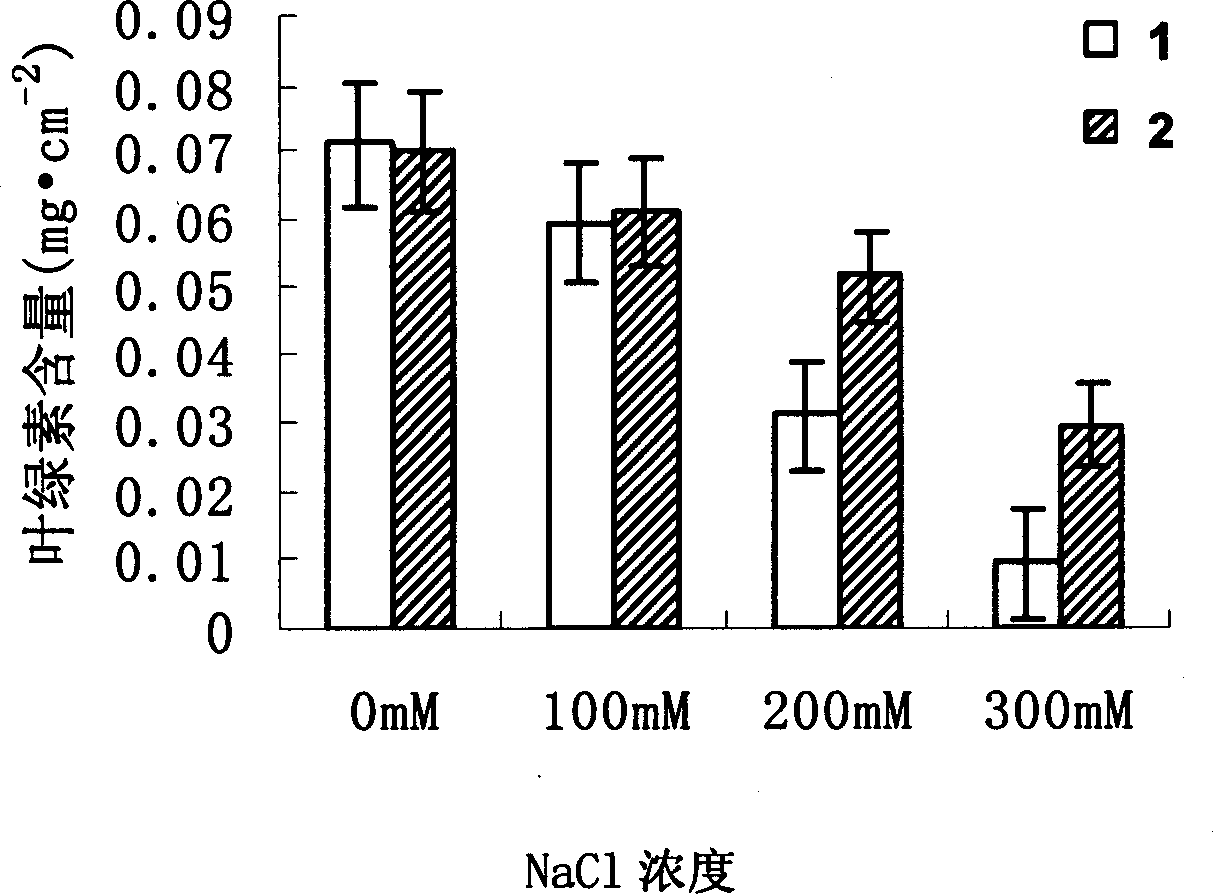
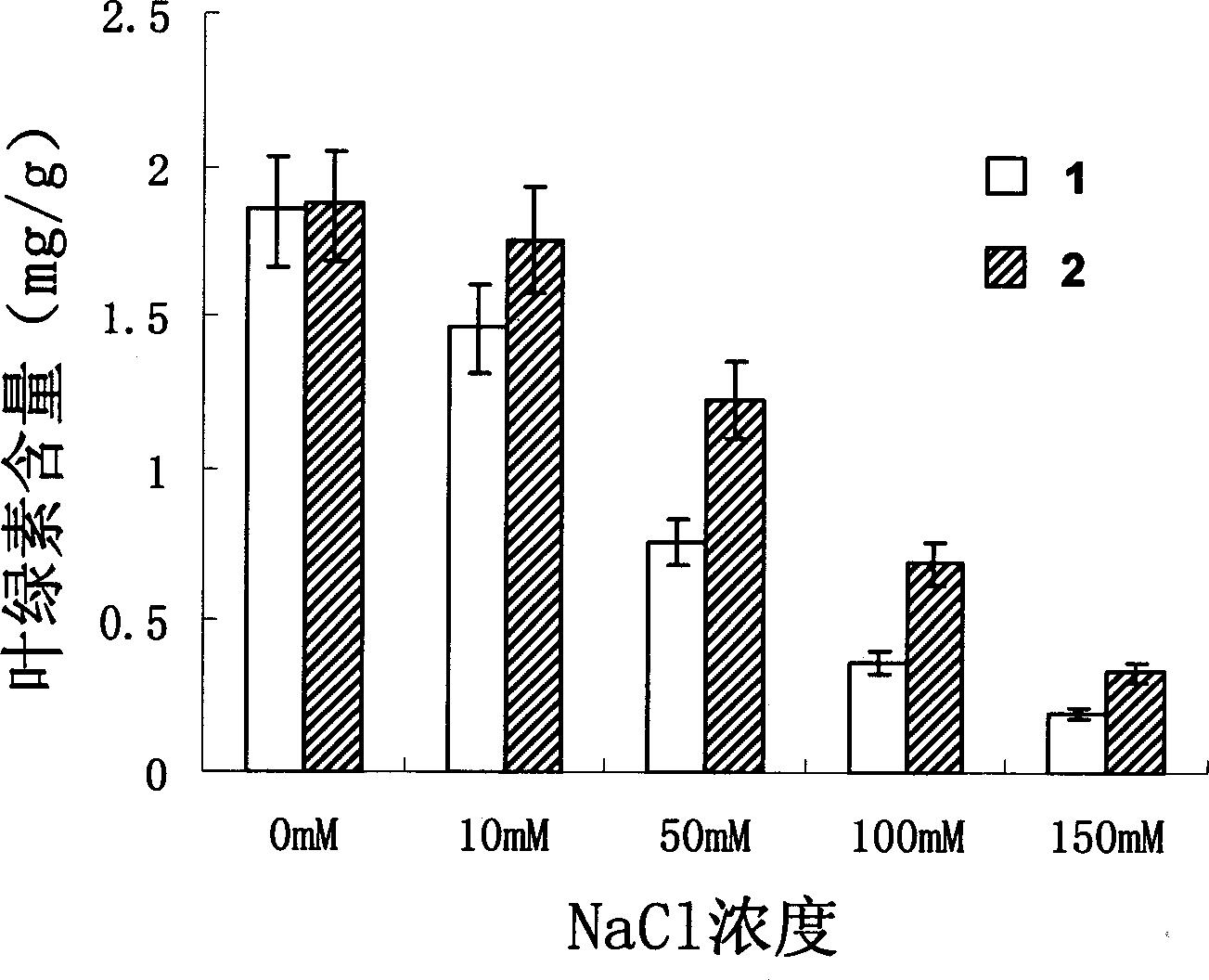
The invention is about the clone, recombination and the salt tolerant analysis of the GhZFP1gene of the zinc finger structure of the CCCH type in the cotton. The invention is to extract the total RNA from the cotton leaves after treating by the 0.3mol / L NaCl to separate the mRNA and construct the cDNA library, then to select the gene encoded the GhZFP1 protein of the zinc finger of the CCCH type. The tobacco transformed by the gene can grow in the concentration of 200mmol / L salt; and the paddy can grow in the in the concentration of 100mmol / L salt. So it can improve the haloduric ability of the plants to plant the plants in the alkaline soil.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Novel coronavirus antigen colloidal gold diagnostic kit
PendingCN111285933AHigh homologyRapid diagnosisPolypeptide with localisation/targeting motifSsRNA viruses positive-senseAntigenProtein antibody
The invention discloses an SARS-COV2 fibrin antibody which is a pairing antibody of an S1 region of SARS-COV2 fibrin. The invention further discloses a preparation method of the SARS-COV2 fibrin antibody. The preparation method of the SARS-COV2 fibrin antibody comprises the following steps: expressing and purifying a gene of an S1 protein Gln14-Arg685 region based on SARS-COV2 to obtain a fusion protein, and then preparing a paired antibody from the fusion protein. The SARS-COV2 fibrin antibody is used for preparing a novel coronavirus detection kit, and the invention provides the novel coronavirus antigen colloidal gold diagnosis kit which comprises the SARS-COV2 fibrin antibody. The SARS-COV2 colloidal gold diagnostic kit provided by the invention is simple to operate, can quickly diagnose whether a patient is infected with SARS-COV2 or not, and is beneficial to effective control of epidemic situations.
Owner:SICHUAN MACCURA BIOTECH CO LTD
Mutant fibroblast growth factor and use thereof in treating endocrine diseases
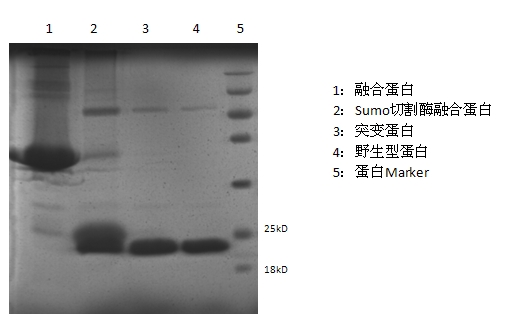
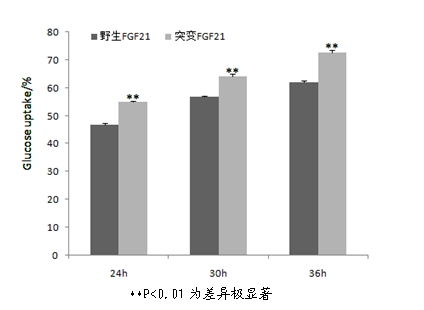
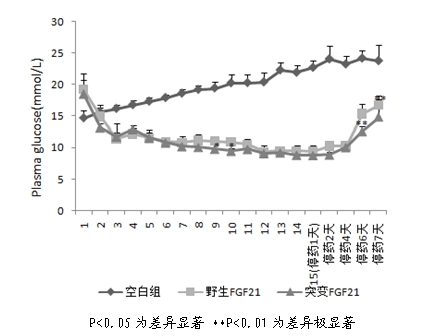
ActiveCN102603886AImprove stabilityQuick effectBacteriaPeptide/protein ingredientsArginineCell membrane
The invention discloses a mutant fibroblast growth factor (FGF-21) and use of the mutant fibroblast growth factor in treating endocrine diseases. The amino acid sequence of the fibroblast growth factor-21 disclosed by the invention is shown as SEQ ID NO: 2, and the sequence of a gene for encoding the mutant fibroblast growth factor is shown as SEQ ID NO: 1. According to the invention, a wild typeFGF-21 is used as a template, two arginine (Arg) residues are introduced through a downstream primer, and a mutant is obtained through polymerase chain reaction (PCR). The strong basicity and positive charges in the physiological condition of Arg are mainly utilized so that the isoelectric point of FGF-21 is up-regulated, and FGF-21 binding to the surfaces of cell membranes is facilitated. The results of an animal experiment show that the mutant FGF-21 disclosed by the invention can more effectively reduce the blood glucose level in an animal, and furthermore, the mutant disclosed by the invention has the advantages of fast onset of drug action, lasting drug effect and the like in reducing the blood glucose level. The mutant GF-21 disclosed by the invention can be used as a medicine to treat endocrine diseases such as diabetes, metabolic syndrome, lipid metabolism disorder and the like.
Owner:TIANJIN TASLY PHARMA CO LTD
DNA fragment for stable expression of an exogenous gene in a plant
InactiveUS6573429B1Stable expressionHigh homologySugar derivativesOther foreign material introduction processesDNA fragmentationGene
Owner:NARA INST OF SCI OF TECH
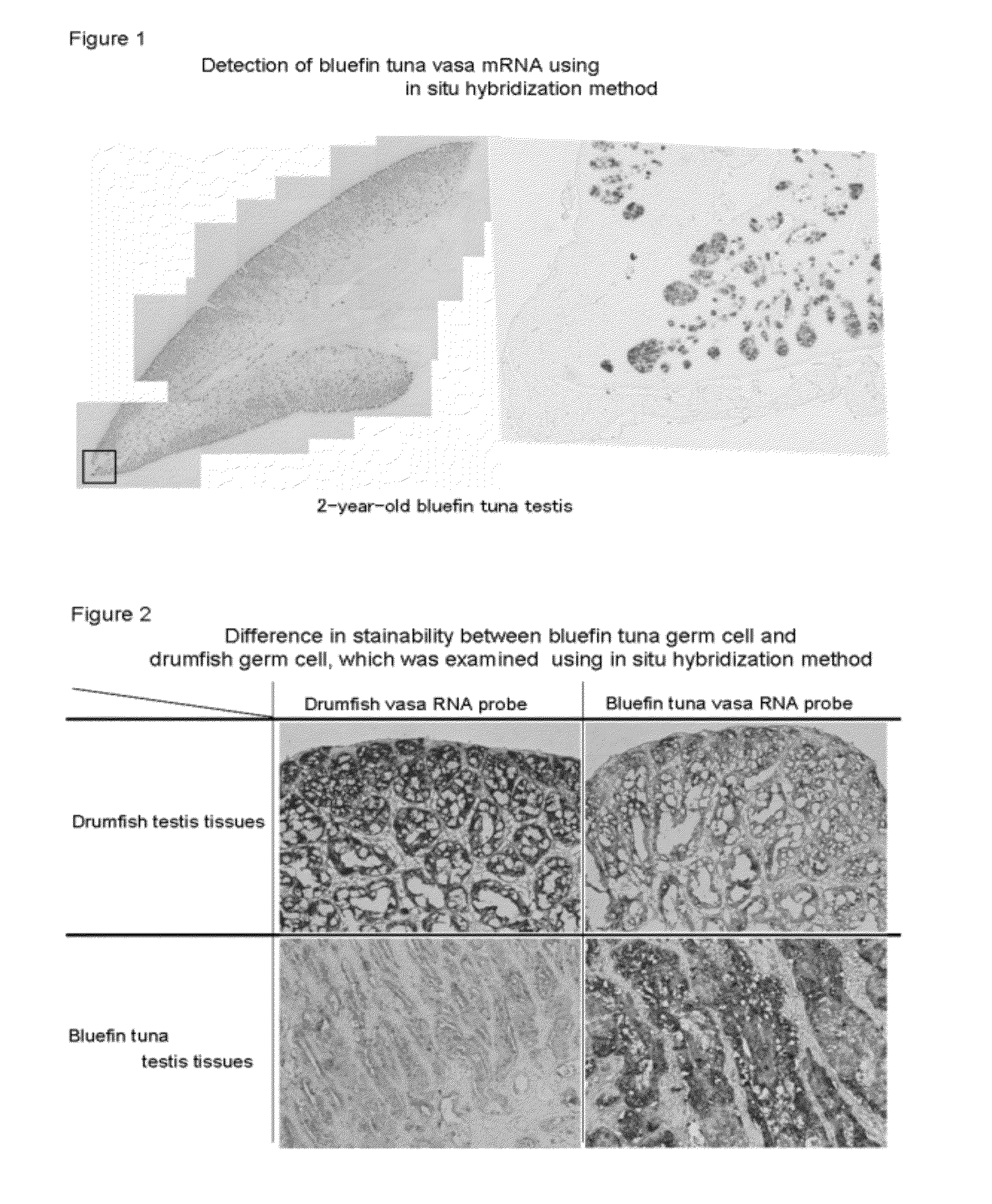
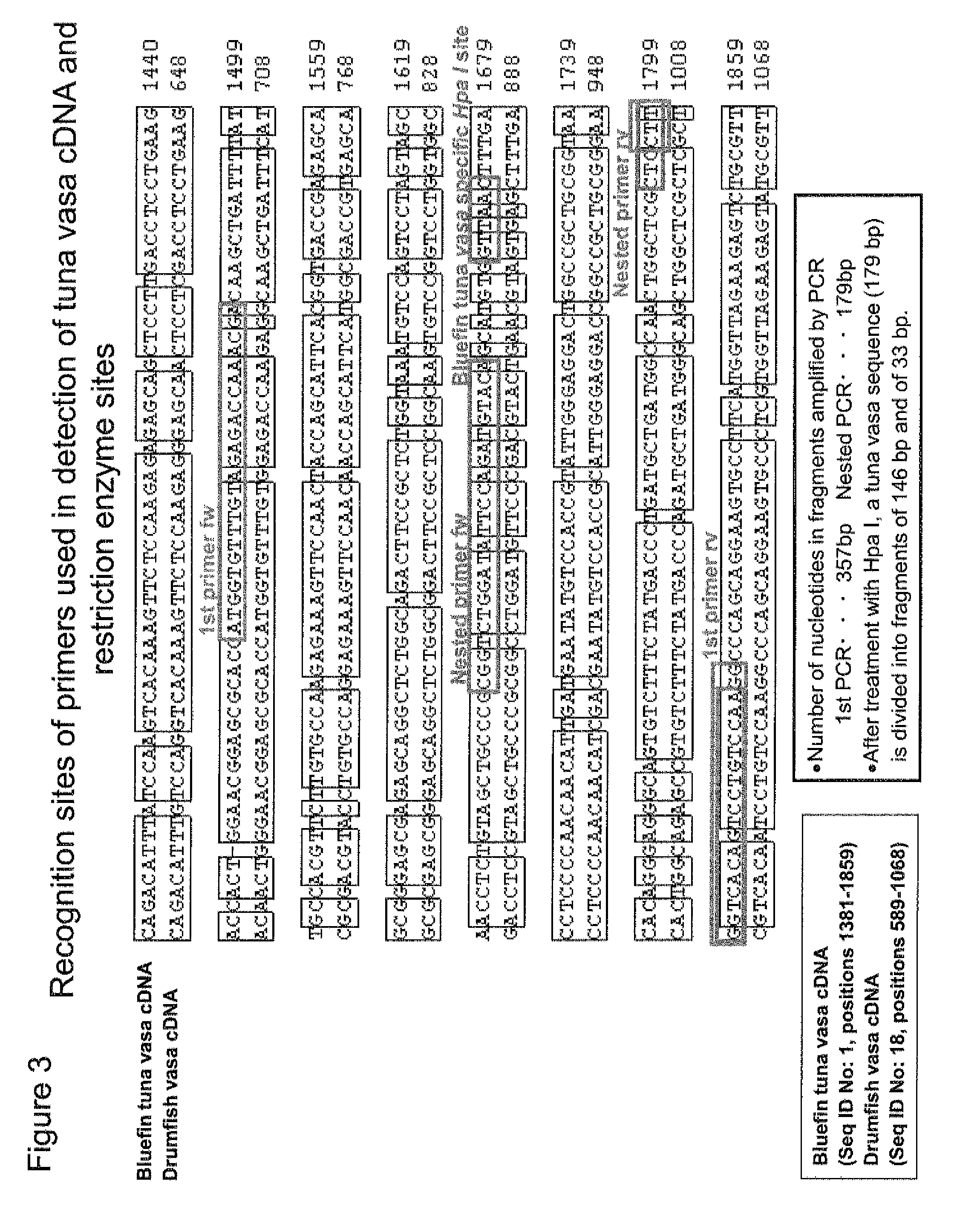
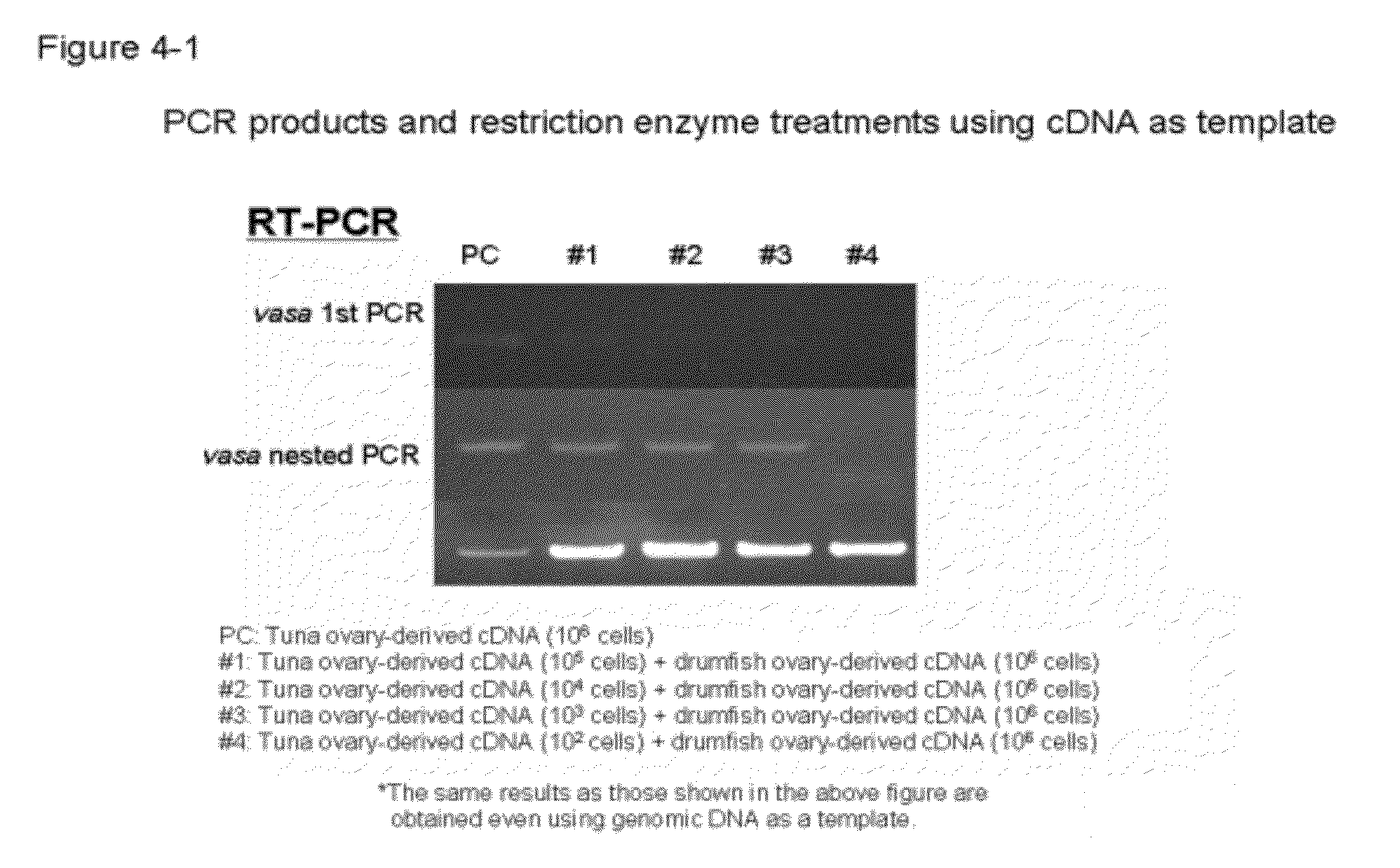
Germ cell marker using fish vasa gene
Owner:NIPPON SUISAN KAISHA LTD
Injectable implant and preparation method thereof
ActiveCN104055795AKeep buildSuppress generationSenses disorderPeptide/protein ingredientsPhosphateActive component
The invention relates to an injectable implant and a preparation method thereof. The implant is amnion microparticles mixed in a sodium chloride phosphate physiological solution with a pH of 7.0-7.5. The preparation method comprises the following steps of disinfection processing, decellularization processing, modification processing, and amnion microparticle preparation, and in particular, comprises: taking amnion microparticles as a component A and a sodium chloride phosphate physiological solution as a component B, performing vibration and well mixing according to a mass volume ratio of 20:1-100:1 to prepare an amnion microparticle suspension with a concentration of 20-100 mg / mL by high-speed microjet equipment, wherein the particle size of the amnion microparticles is 50-200 microns. The injectable implant of the invention maintains the natural three dimensional stereo structure and a lot of natural active components of human amnion, reduces immunological rejection reaction caused by raw material source after clinical application of cosmetic injection products, is suitable for injection into deep subcutaneous dermal layer to subcutaneous superficial layer to repair moderate to severe wrinkles or folds, and has good cosmetic repair effect.
Owner:SHAANXI RUISHENG BIOTECH
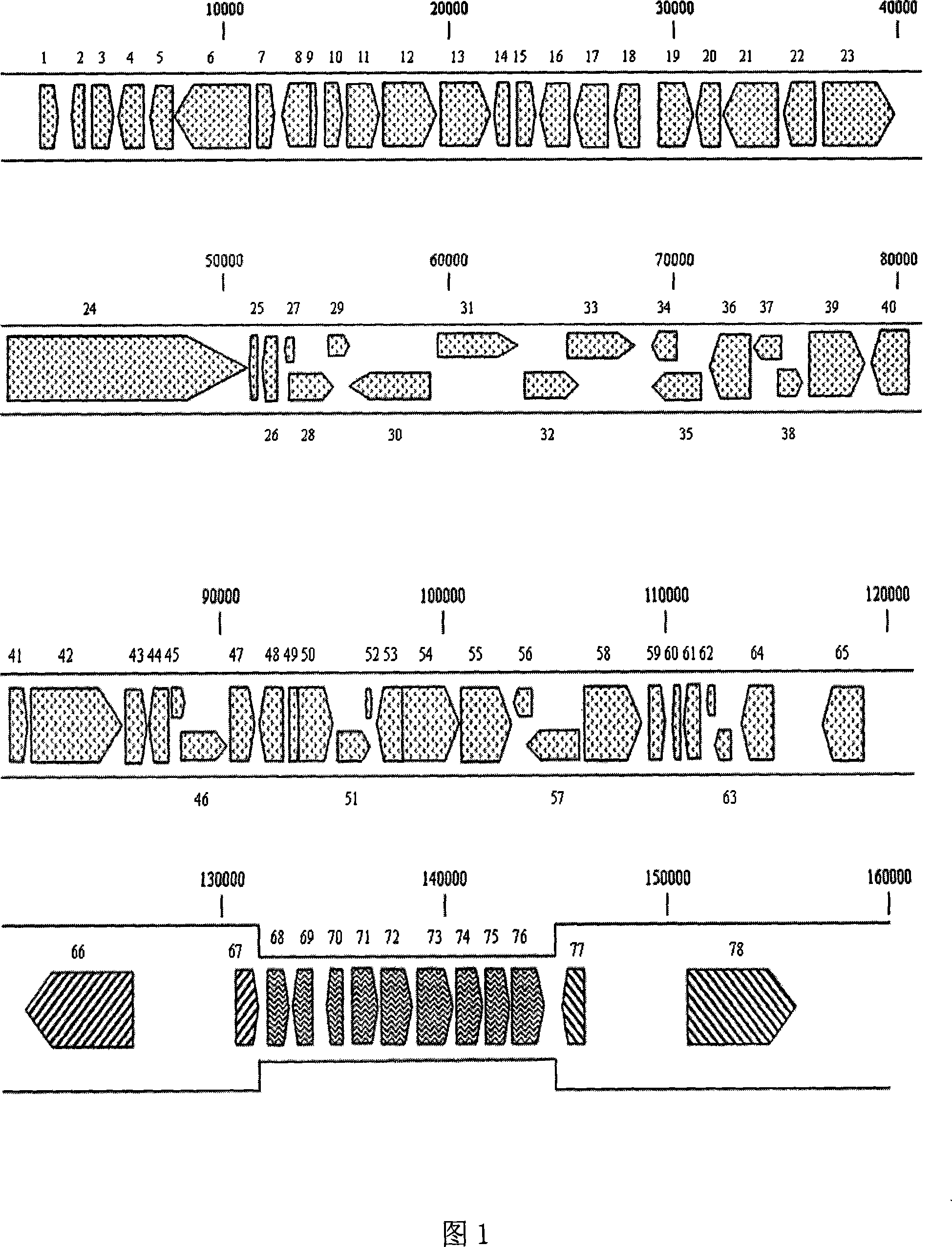
Extraction of duck enteritis virus genom DNA and sequence thereof
InactiveCN101182526AHigh homologyShort copy cycleFermentationGenetic engineeringPUC19Escherichia coli
The invention discloses a DNA extraction method of Duck Enteritis Virus genome and the genome sequence. Firstly, the pyrolysis of chicken embryo fibroblast which is infected with DEV is processed by hypertonic buffer; then the nuclease is added for the digestion of cell DNA; and the virus genome DNA is obtained through phenol chloroform extracting method. The obtained sample is broken randomly into 2kb to 2.5kb fragments; the pUC19 vector is linked to transform host E. coli and construct genomic library. The positive clones in the library are randomly sequenced; the total sequence length has 6 times coverage rate for the virus genome. The sequence result is assembled and clustered to obtain the whole DEV genome sequence with the length of156512bp. The analysis shows that the genome structure is the same with that of the members of Varicella virus in Alpha- herpesus subfamily, which provides theoretical basis for the classification of DEV.
Owner:POULTRY INST SHANDONG ACADEMY OF AGRI SCI
Artificial recombined hexon protein A, constructing method thereof and use
InactiveCN101337986AEasy to purifyExcellent potential activityBacteriaDepsipeptidesGenetic engineeringProtein A
The invention belongs to the technical field of genetic engineering, and relates to recombinant hexamer protein A as well as the constitution method and the application thereof. The recombinant hexamer protein A has an amino acid sequence represented by SEQ ID NO.1 in a sequence list. The recombinant hexamer protein A has higher activity than those of the reported recombinant protein A and the natural protein A, and has high yield and relatively simple purification process, thereby preventing the risk of pathogenic bacteria of naturally extracted protein. Additionally, the recombinant hexamer protein A suits large-scale production.
Owner:GUANGZHOU KONCEN BIOSCI
Method of detecting mutation and kit used in the same
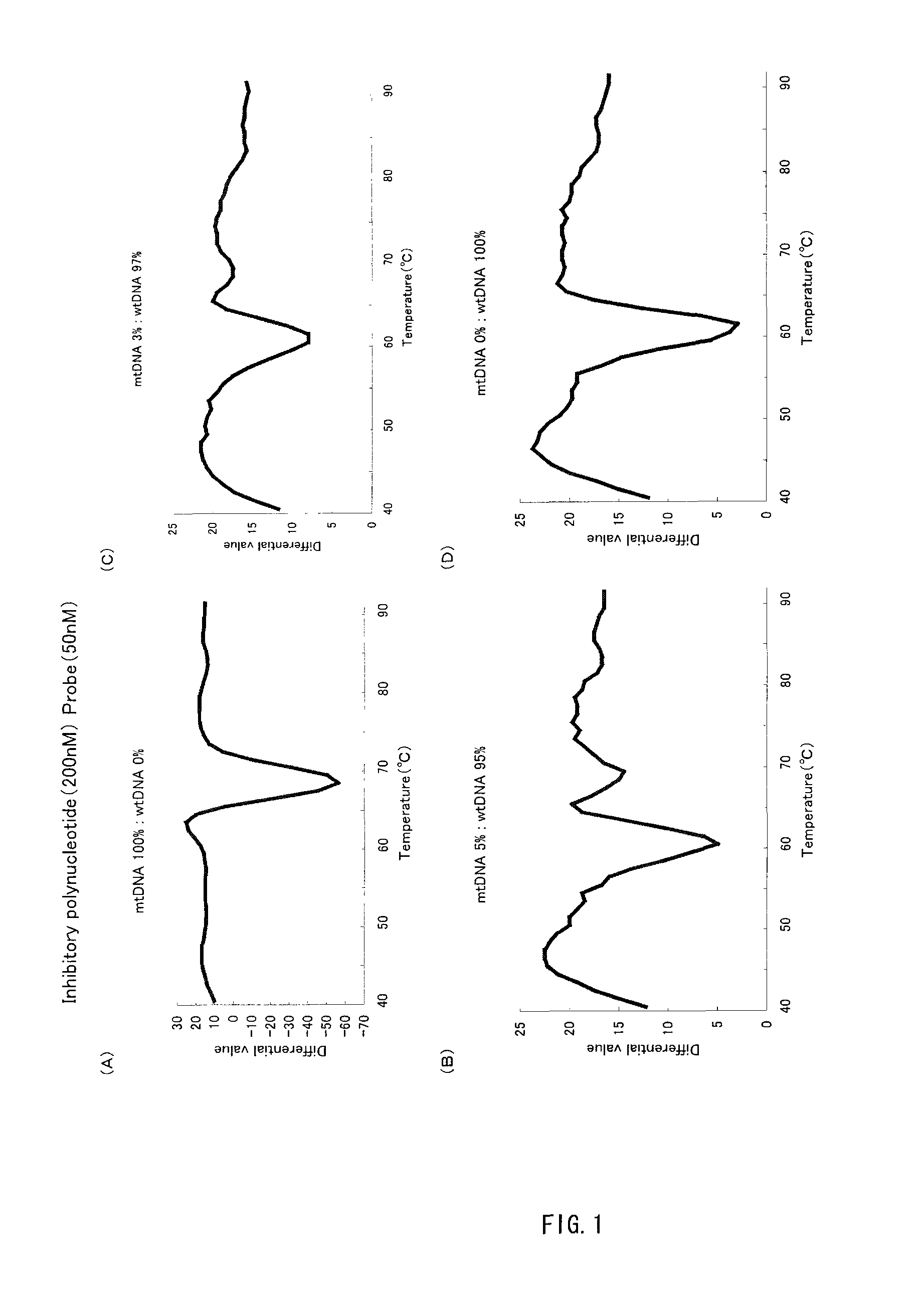
InactiveUS20100216123A1Easy to carryHigh sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsMutation detectionA-DNA
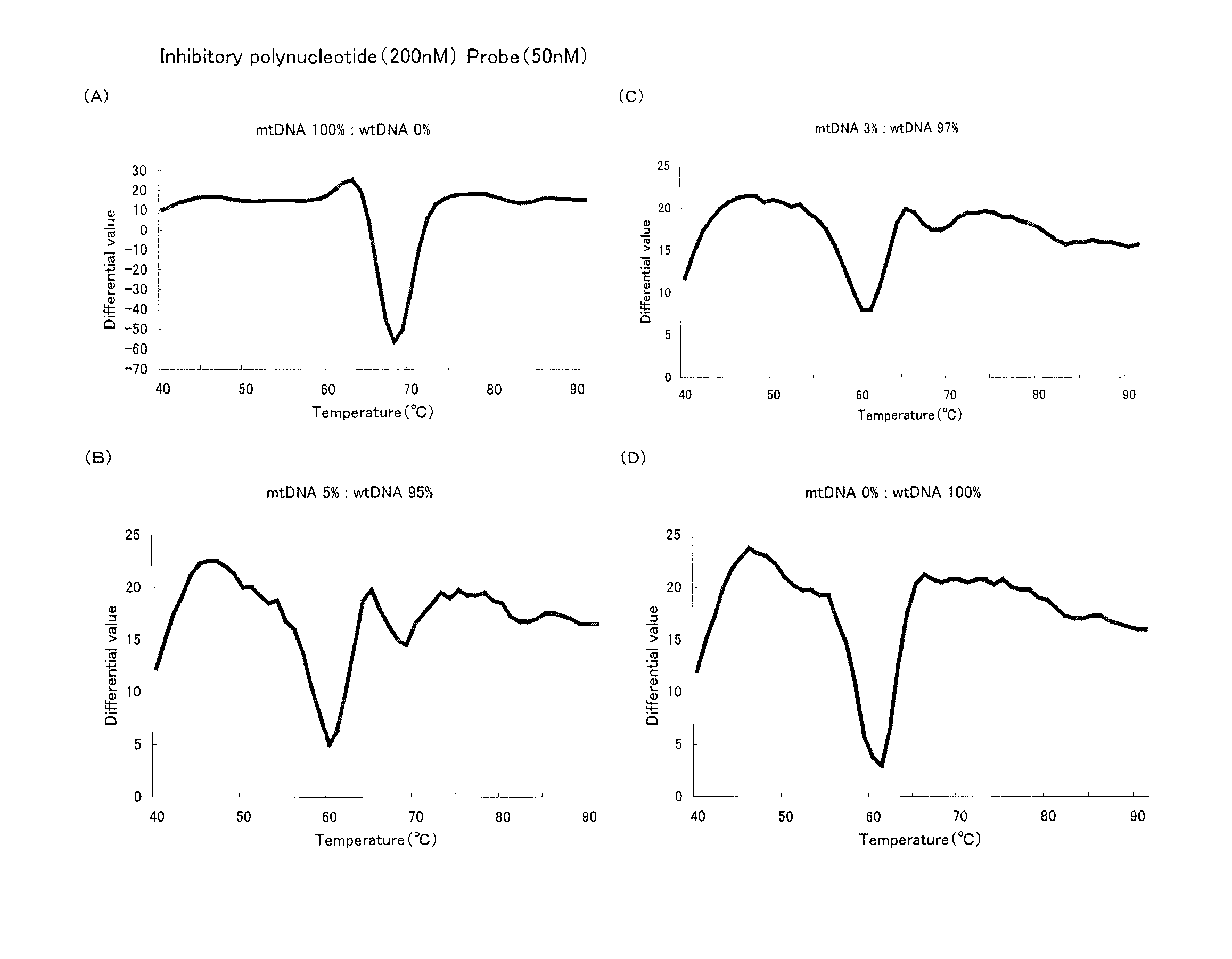
A method of detecting a mutation is provided that uses Tm analysis and is excellent in detection sensitivity. A detection probe consisting of a polynucleotide complementary to a sequence to be detected containing a detection site that has been mutated and an inhibitory polynucleotide complementary to a sequence not to be detected containing the detection site that is unmutated are added to a sample containing a DNA to be detected in which the detection site has been mutated and a DNA not to be detected in which the detection site is unmutated, so that the detection probe is hybridized with the DNA. Then while the hybridization product between the DNA and the detection probe is heated, a signal variation associated with an increase in temperature is measured, then the signal variation is analyzed, and thereby a Tm value is determined, based on which the presence of the mutation is determined.
Owner:ARKRAY INC
Pangolin coronavirus xCoV and application thereof and application of medicine in resisting coronavirus infection
ActiveCN113046327AHigh homologyProof of InhibitionSsRNA viruses positive-senseMicrobiological testing/measurementReceptorCepharanthine
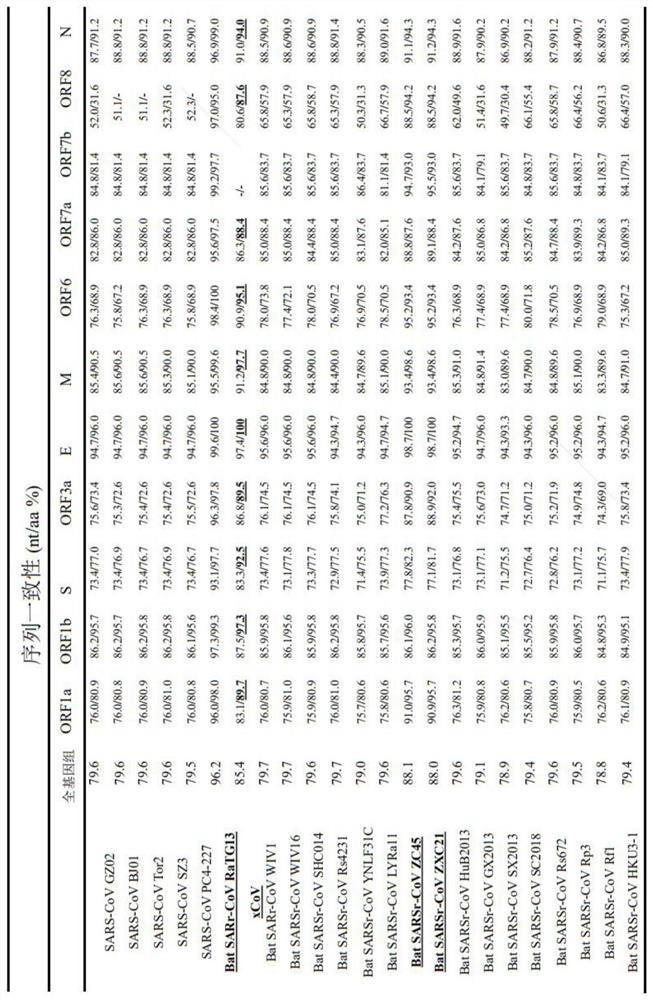
The invention provides a coronavirus separated from pangolin. The coronavirus is named as pangolin coronavirus xCoV, the homology of the coronavirus xCoV and S protein of SARS-COV-2 reaches 92.5%, receptors of xCoV infected cells and the SARS-COV-2 are consistent and are angiotensin converting enzyme 2 (ACE2). However, the xCoV does not infect people, is very safe to people, can be used for screening active drugs and vaccines for resisting the SARS-COV-2 virus, and can also be used for preparing attenuated vaccines or inactivated vaccines for resisting the SARS-COV-2 virus. Based on the pangolin coronavirus xCoV, a plurality of active compounds with anti-coronavirus activity are obtained by screening, EC50, CC50 and SI evaluation is performed on cepharanthine (stephanine), silamectin and mefloquine hydrochloride (mefloquine) in the active compounds, and the xCoV inhibition mechanism of cepharanthine is analyzed and researched through transcriptome sequencing.
Owner:BEIJING UNIV OF CHEM TECH
Use of hemagglutinin of the african swine fever virus as an adjuvant
InactiveUS20100086556A1Enhance immune responseHigh homologyOrganic active ingredientsVirusesHemagglutininAdjuvant
The invention generally relates to the use of the hemagglutinin (HA) of African swine fever virus (ASFV) as an adjuvant to enhance the immune response against an antigen in a subject. The invention provides a gene construct comprising all or part of the encoding sequence of said HA fused to the encoding sequence of an antigen. The invention is applicable in human and animal health.
Owner:FUNDACIO CENT DE RECERCA & SANITAT ANIMAL
Rice chlorophyll synthase mutant gene and its application in gene engineering
InactiveCN101096676AHas chlorophyll synthase activityHigh homologyEnzymesVector-based foreign material introductionAgricultural scienceNucleotide
The invention discloses a nucleotide sequence of rice chlorophyll synthase mutation gene Osygl1 and gene engineering application in the gene engineering domain, which is characterized by the following: the cDNA sequence of Osygl1 is SEQ ID NO.1 and its coded amino acid sequence is SEQ ID NO.2; the gene OsYGL1 is a chlorophyll synthase gene in the rice, which participates the synthesis of chlorophyll a and chlorophyll b to display component expression; the mutation of the gene leads the leaf of rice to become yellow green, which can be genetic mark to identify the kind in the agricultural manufacturing and rice genetic breeding.
Owner:NANJING AGRICULTURAL UNIVERSITY
Biological repair tablet for endocranium and preparation method thereof
The invention provides a biological repair tablet for endocranium and a preparation method thereof. The repair tablet uses small intestine submucosa tissues of inbred line animals without cell and DNA (deoxyribonucleic acid) components as the raw material, completely reserves the extracellular matrix component and structure, and has a micropore structure. The preparation method of the repair tablet comprises the following operation steps: determination of animal source, pretreatment and rough cleaning of small intestine tissues, virus inactivation, cell removal, DNA removal treatment, formation, packaging and sterilization. The biological repair tablet for endocranium prepared by the method uses an inbred line animal as an animal source, and thus, the hereditary features are pure, stable and uniform, thereby radically ensuring the stability and uniformity of different batches of products; and the biological repair tablet for endocranium has fewer animal source DNA residues, completely reserves the three-dimensional structure of natural ECM, and has the advantages of low immune source property and high infection resistance.
Owner:BEIJING BIOSIS HEALING BIOLOGICAL TECH
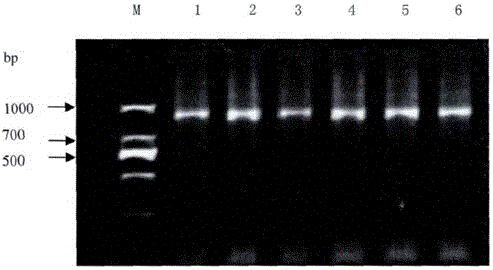
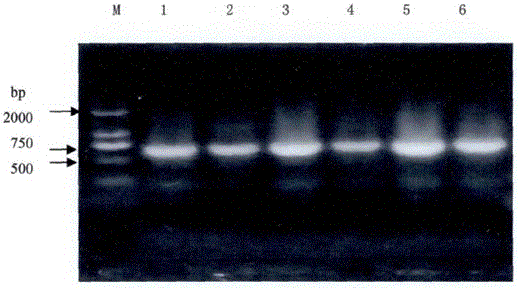
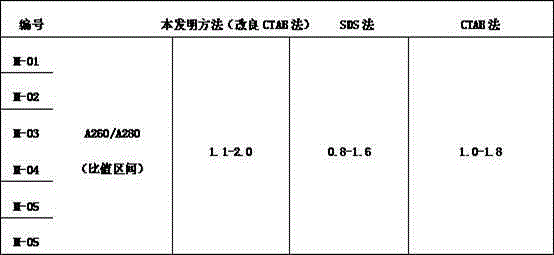
Improved CTAB method for extracting polygonum capitatum DNA
The invention relates to an improved CTAB method for extracting polygonum capitatum DNA. The method comprises the following steps: fetching polygonum capitatum, grinding polygonum capitatum into powder in liquid nitrogen, transferring the powder to a centrifuge tube, adding a CTAB extracting buffering solution, fully shaking the centrifuge tube, carrying out water bath and centrifugation; obtaining the supernate, adding chloroform and isoamyl alcohol with an equal volume, transferring the supernate to another centrifuge tube, collecting the supernate again, extracting the supernate by chloroform and isoamyl alcohol; adding NaAc and isopropanol into supernate, slightly turning over the centrifuge upside down to evenly mix the solution, carrying out centrifugation, discarding the supernate, washing the precipitate by ethanol (70%), carrying out centrifugation, absorbing ethanol by a small gun head, drying the precipitate in the air; removing the ethanol in precipitate, dissolving and digesting the precipitate by TE containing RNA enzymes until the RNA is completely degraded; extracting the solution by phenol, chloroform, and isoamyl alcohol with an equal volume respectively, adding supernate into NaAc and cold anhydrous ethanol, slightly and evenly mixing, carrying out centrifugation, washing the precipitate by an ethanol solution (70%), drying the precipitate in the air, adding a TD solution to dissolve the precipitate, and finally preserving the solution at a low temperature. The modified CTAB method is used to extract polygonum capitatum DNA and has the advantages of simpleness, low cost, and higher purity of polygonum capitatum DNA.
Owner:AFFILIATED YONGCHUAN HOSPITAL OF CHONGQING MEDICAL UNIV
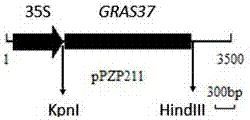
Maize gene ZmGRAS37 for regulating and controlling nutrient body largeness, early blossoming and grain weight increment of arabidopsis thaliana at seedling stage and application thereof
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Generation of tumor-free embryonic stem-like pluripotent cells using inducible recombinant rna agents
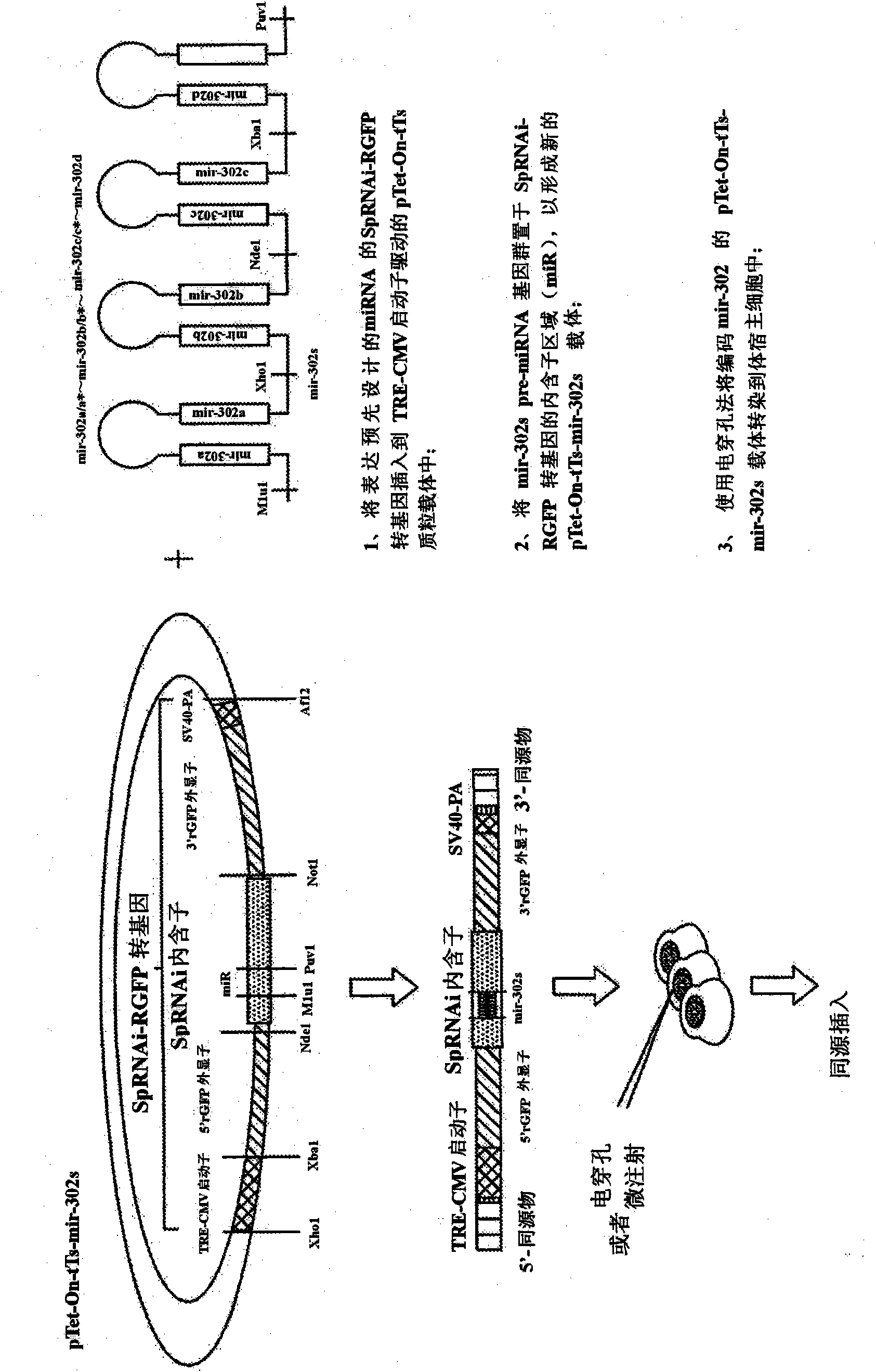
ActiveCN101970664AIncrease translation efficiencyStrong gene silencing effectGenetically modified cellsCell culture active agentsCancer cellMammal
The present invention generally relates to a method for developing, generating and selecting tumor-free embryonic stem (ES)-like pluripotent cells using electroporation delivery of an inducible tumor suppressor mir-302 agent into mammalian cells. More particularly, the present invention relates to a method and composition for generating a Tet-On / Off recombinant transgene capable of expressing a manually re-designed mir-302 microRNA (miRNA) / shRNA agent under the control of doxycyclin (Dox) in human somatic / cancer cells and thus inducing certain specific gene silencing effects on the differentiation-associated genes and oncogenes of the cells, resulting in reprogramming the cells into an ES-like pluripotent state.
Owner:林希龙 +1
Indirect ELISA (enzyme-linked immuno sorbent assay) kit for detecting haemophilus parasuis antibody
ActiveCN103941020AHigh homologyImproved conservatismBiological material analysisBiological testingToxin proteinCytolethal distending toxin
The invention discloses an indirect ELISA (enzyme-linked immuno sorbent assay) kit for detecting a haemophilus parasuis antibody. The kit consists of an ELISA coating plate with haemophilus parasuis cytolethal distending toxin)-C protein serving as a coating antigen, a to-be-detected sample dilution plate, a positive contrast serum, a negative contrast serum, 20-time concentrated washing liquid, a serum sample dilution solution, an enzyme-labeled antibody working solution, a developing solution and a terminating solution. A judgment standard is that if an S / P value is less than 0.200, a sample to be detected is negative; if the S / P value is greater than or equal to 0.200, the to-be-detected sample is positive; the S / P value is obtained according to a formula: S / P value=(the mean value of the to-be-detected sample OD450nm-the mean value of a negative contrast OD450nm) / (the mean value of a positive sample OD450nm-the mean value of the negative contrast OD450nm). Due to the specificity test, the sensitivity test, the repetitiveness test, the coincidence rate test, the test for comparing the kit disclosed by the invention with a kit on sale, the clinical application test and the like, the kit disclosed by the invention has the characteristics of high specificity, high sensitivity, high repetitiveness and the like and is high in coincidence rate to the same type of products on sale home and abroad; the indirect ELISA kit can be used for clinical large-scale detection and epidemiological investigation for the haemophilus parasuis antibodies.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
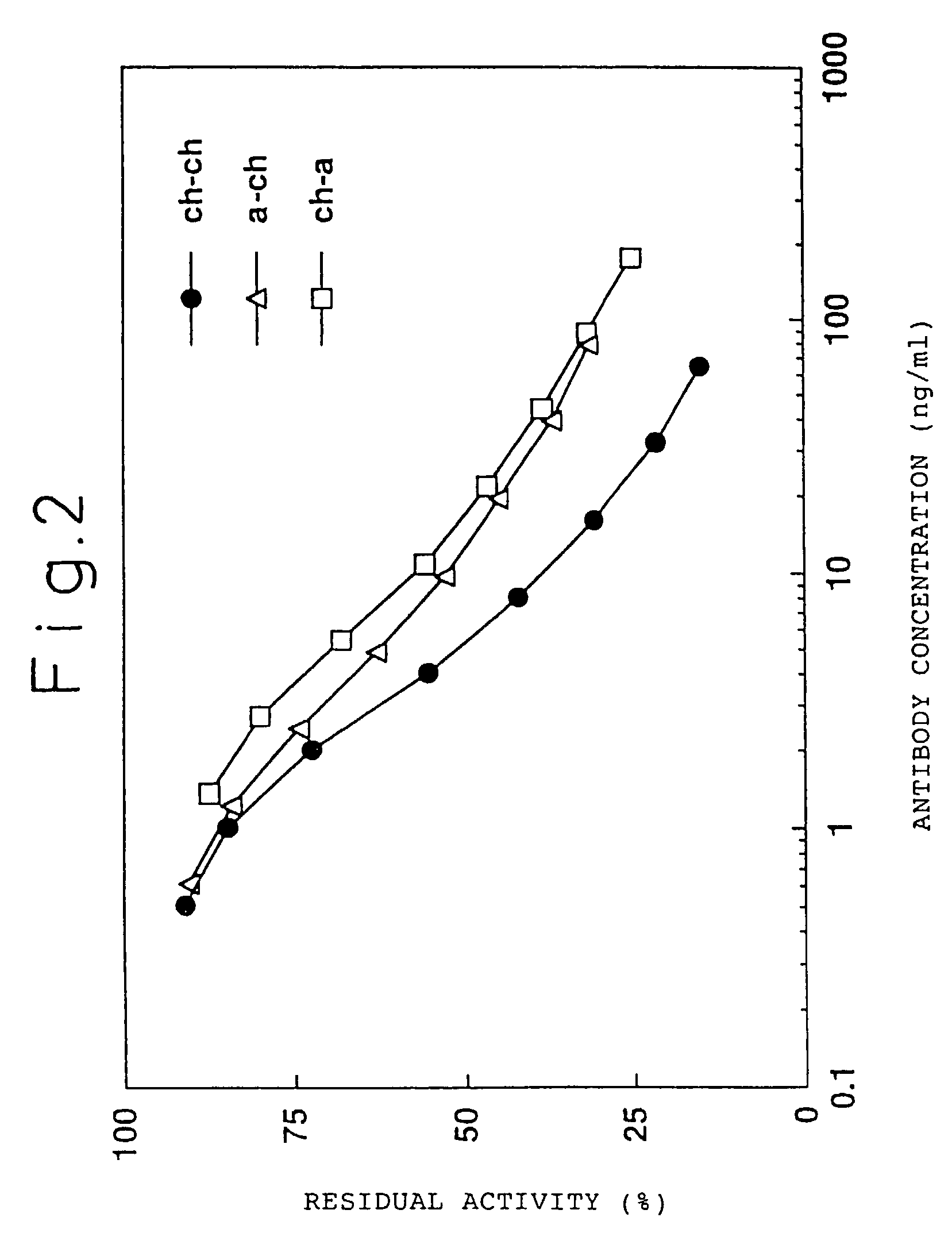
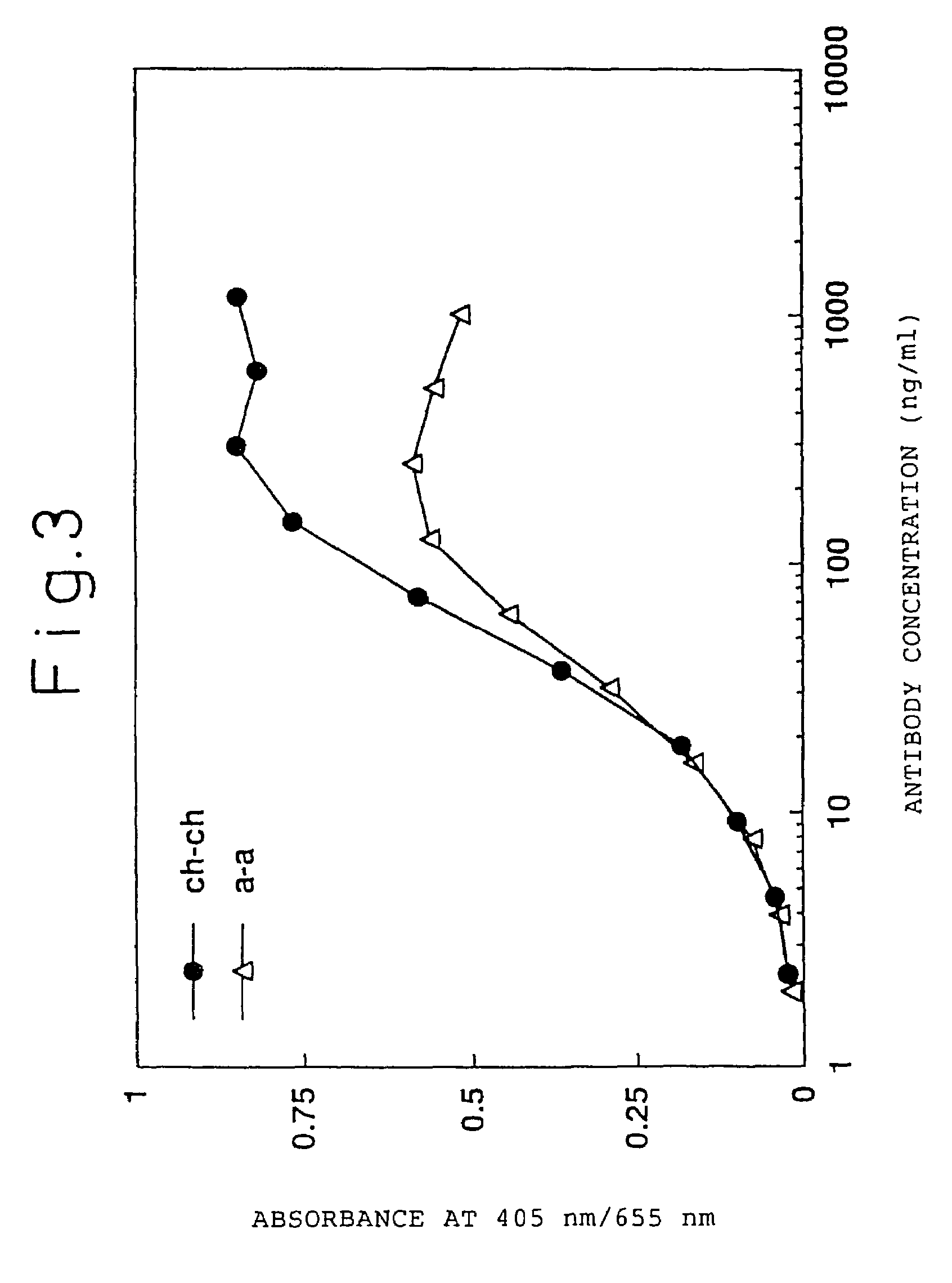
Humanized antibody against human tissue factor (TF) and process of production of the humanized antibody
InactiveUS7494647B2High homologyLow antigenicityImmunoglobulins against blood coagulation factorsAntibody mimetics/scaffoldsMouse monoclonal antibodyHigh homology
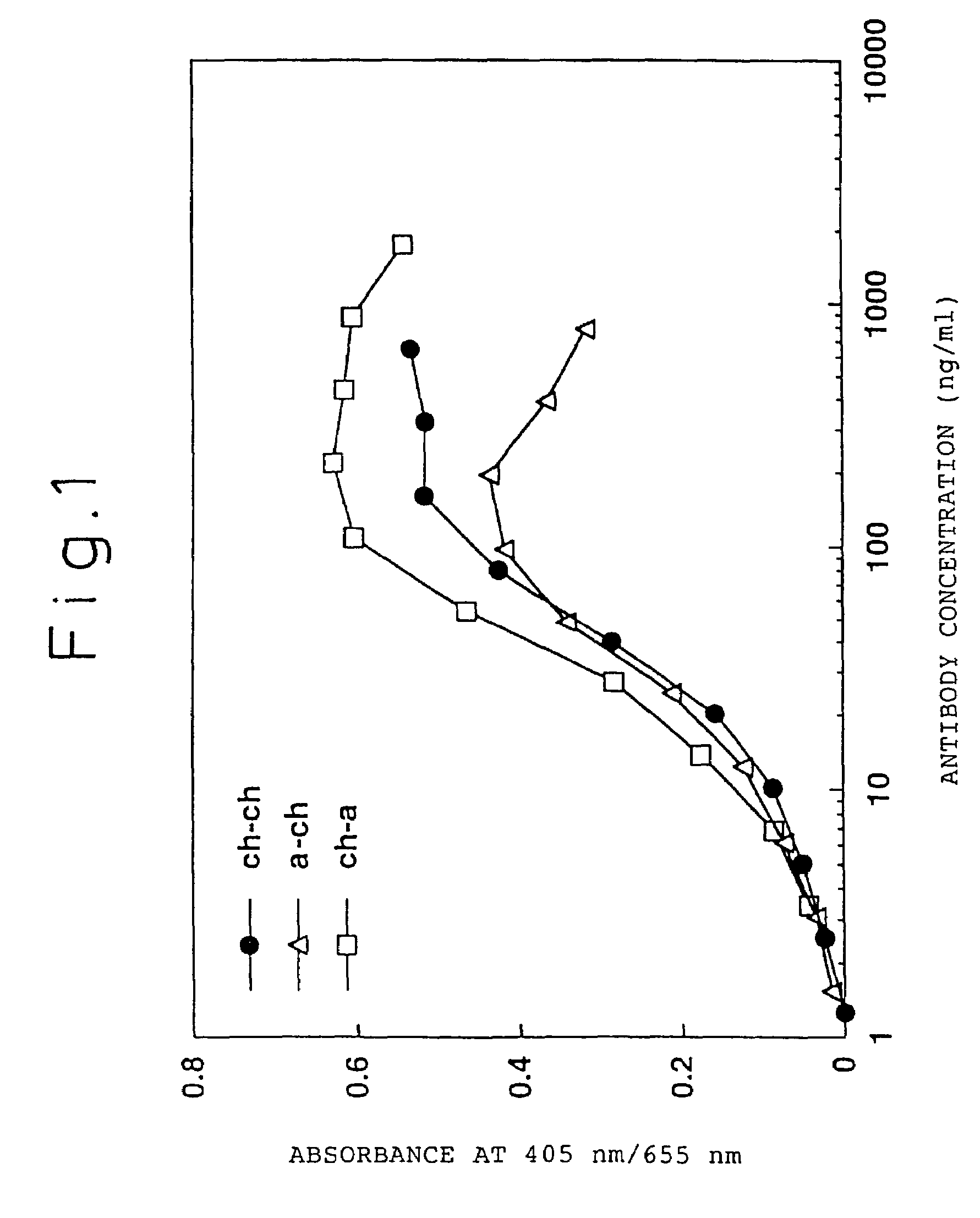
A humanized antibody against tissue factor (TF), comprising:A: a humanized H chain comprising (1) a H chain V region comprising the H chain CDRs of a mouse monoclonal antibody against TF and the H chain FRs of a human antibody, and (2) the H chain C region of a human antibody; andB: a humanized L chain comprising (1) an L chain V region comprising the L chain CDRs of a mouse monoclonal antibody against TF and the L chain FRs of a human antibody, and (2) the L chain C region of a human antibody.After generating a humanized V region by grafting the CDRs of a mouse monoclonal antibody onto a human antibody, a humanized antibody having a higher activity is searched by replacing the FR of the above region with the corresponding FR of the human antibody having a high homology.
Owner:CHUGAI PHARMA CO LTD
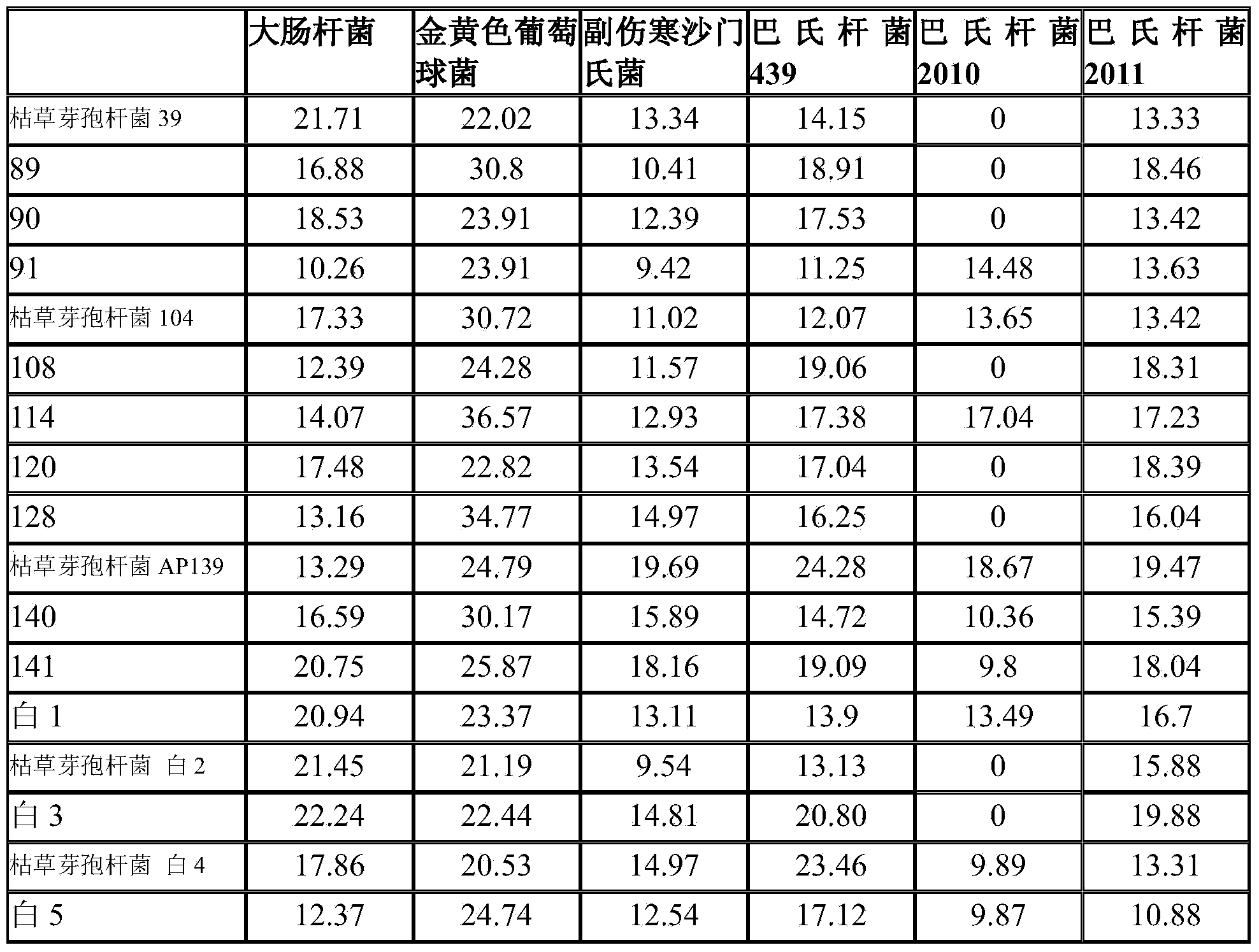
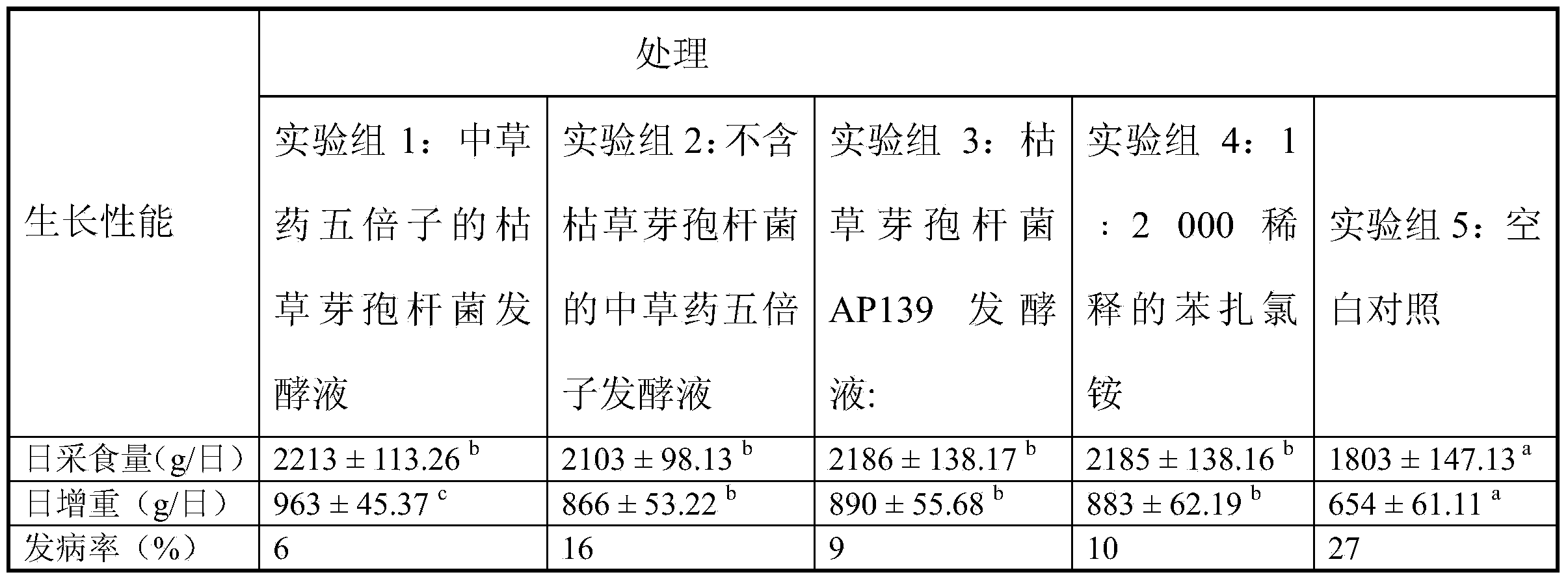
Bacillus subtilis fermentation broth of Chinese herbal medicine as well as preparation and application of bacillus subtilis fermentation broth
InactiveCN104351472AReduce morbidityHarm reductionAntibacterial agentsBiocideStaphylococcus cohniiStaphylococcus aureus
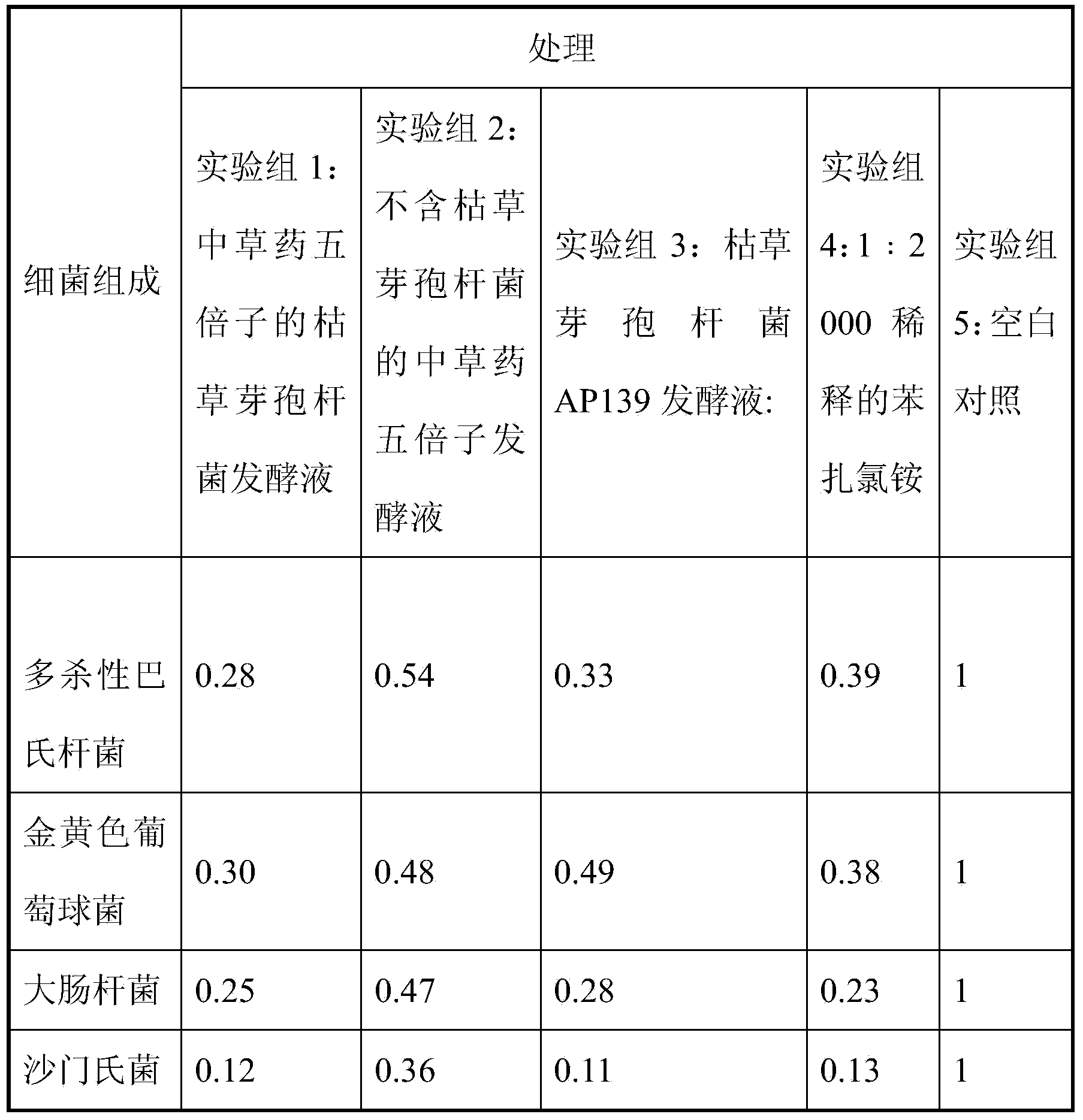
The invention relates to bacillus subtilis fermentation broth of a Chinese herbal medicine as well as preparation and an application of the bacillus subtilis fermentation broth. The Chinese herbal medicine refers to Chinese gall; according to pasteurella multocida and staphylococcus aureus inhibiting screening tests, the Chinese gall can inhibit growth of the pasteurella multocida and the staphylococcus aureus, bacillus subtilis AP139 is used for fermenting the Chinese gall, spraying is performed in an animal breeding shed, the fermented Chinese herbal medicine remarkably inhibits the growth of the pasteurella multocida and the staphylococcus aureus in the air, the effect is superior to those of a bacillus subtilis AP139 separate fermentation group and a Chinese herbal medicine control group, and the fermentation broth has the broader application prospect.
Owner:HUNAN INST OF MICROBIOLOGY
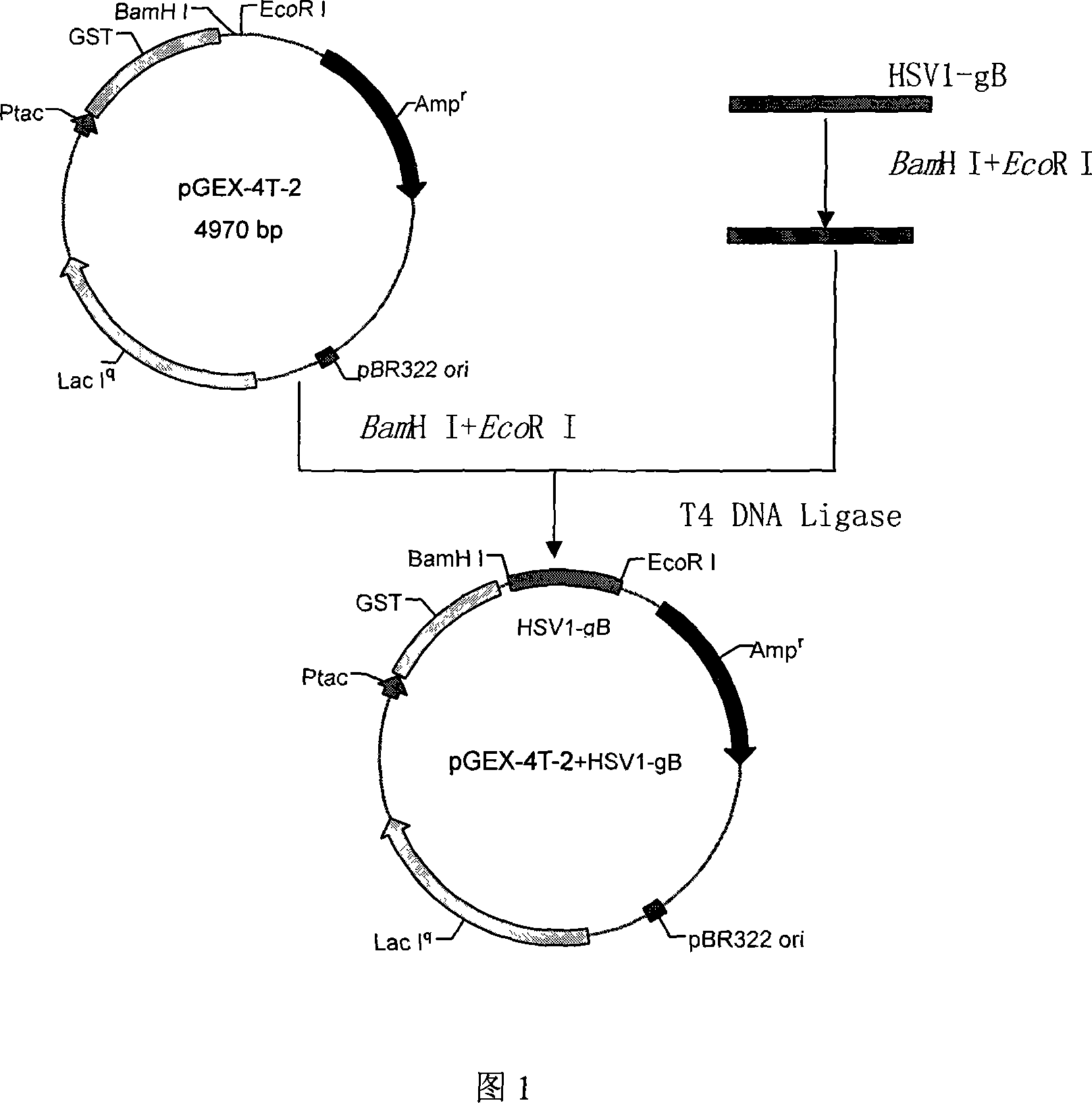
Chemically synthesized HSV1 virus gB glucoprotein extracellular region gene fragment, representation and application of the same
InactiveCN101173290APromote safe productionLow costGenetic material ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsGenetic engineeringGlycoprotein
The chemically synthesized HSV1 viral gB glycoprotein extracellular region gene fragment and its expression and application relate to the fields of genetic engineering technology, vaccines and diagnostic reagents. The present invention screens out the strong epitope in the gB glycoprotein of HSV1 virus through computer analysis, from the first amino acid to the 696th amino acid, a total of 696 amino acids, selects codons favored by both eukaryotic and prokaryotic organisms, and chemically synthesizes The brand-new gene sequence of the antigenic epitope uses genetic engineering technology to express the gene fragment and prepare a strong antigenic epitope fragment of the gB glycoprotein of the HSV1 virus. The expressed strong antigenic epitope fragment of gB glycoprotein of HSV1 virus can be used for the detection of vaccine, HSV1 virus antibody or antigen, and for immunization preparation of anti-HSV1 virus monoclonal antibody and polyclonal antibody and the like.
Owner:李越希
Methods for representing sequence-dependent contextual information present in polymer sequence and uses thereof
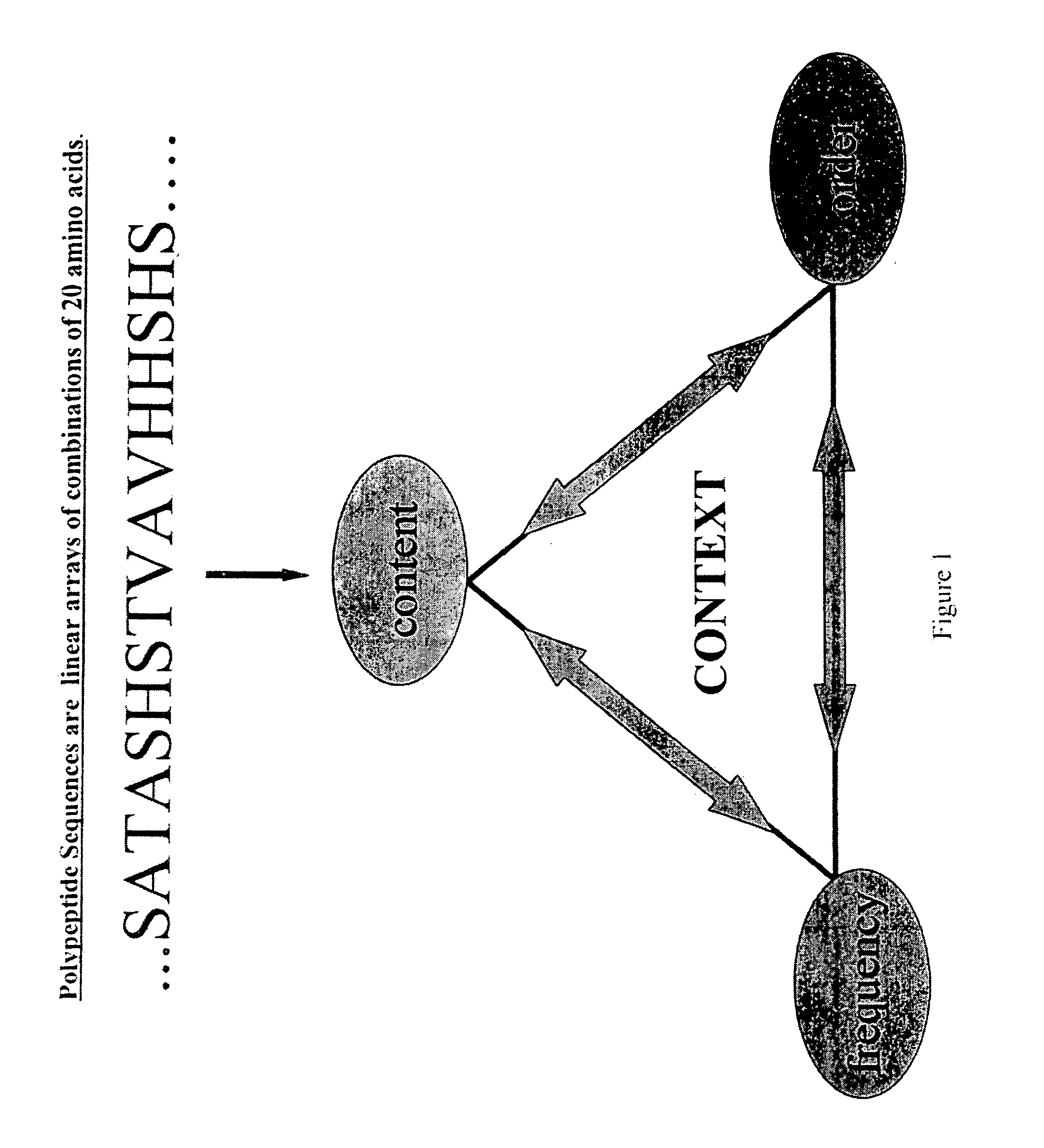
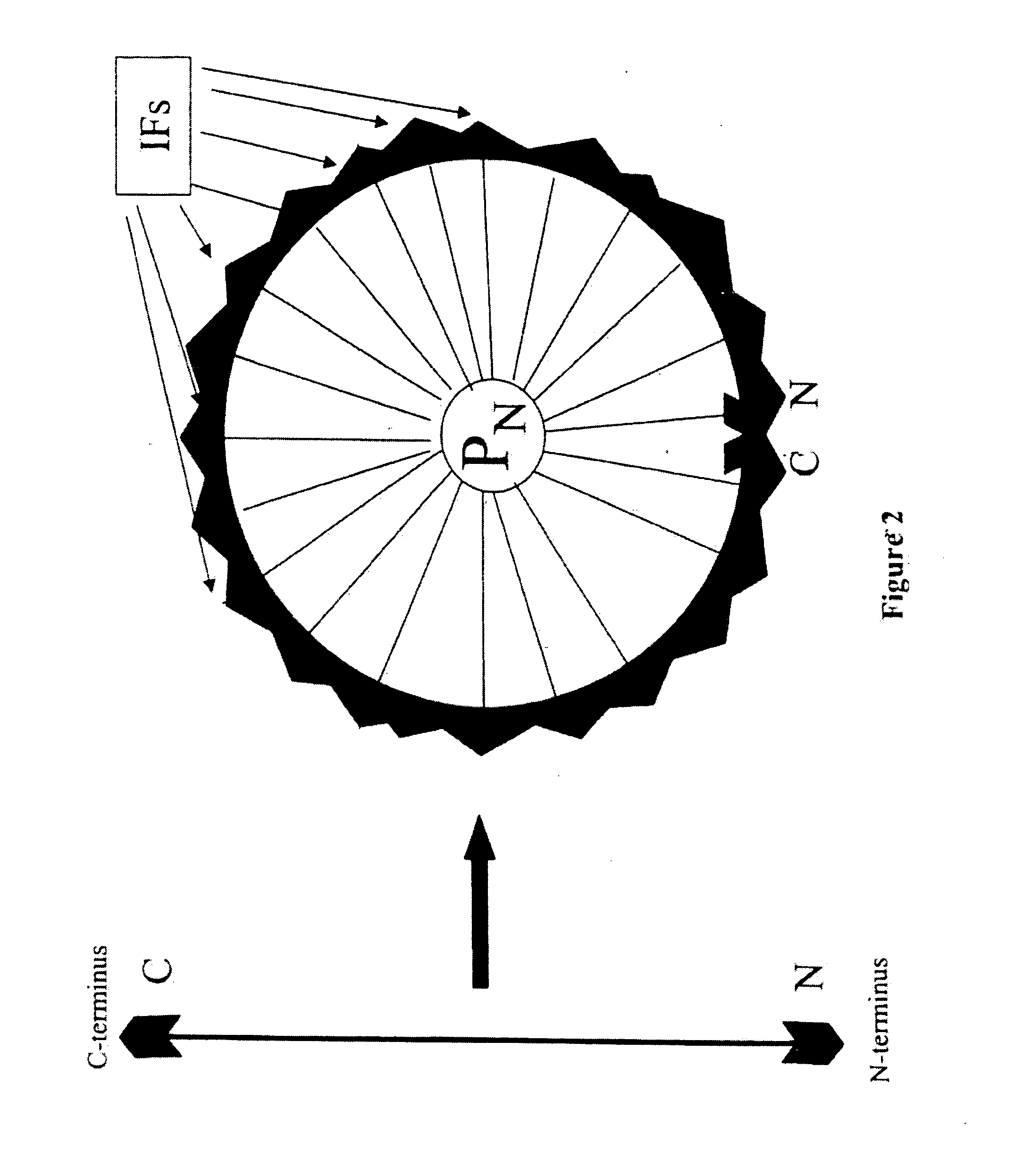
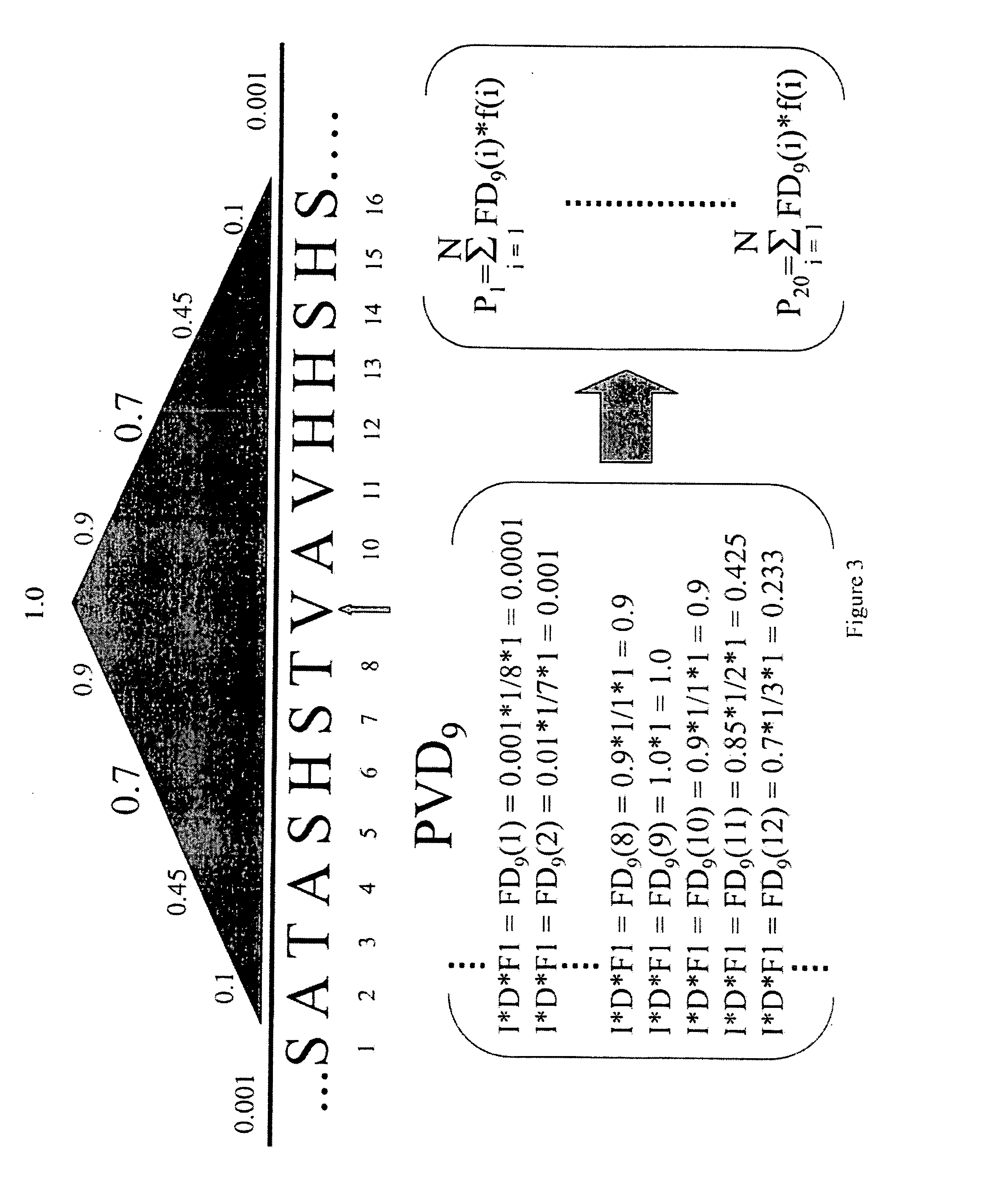
InactiveUS20070192034A1High homologyHigh structural similarityNervous disorderComputing modelsPolymer scienceNucleic acid sequencing
The invention includes methods of representing polymer sequences in a way that reveals important position-specific contextual information. The representations can be used to determine a number of properties of polymers, such as protein and nucleic acid sequences, including the identification of secondary domain structures, folding rate constants, and the effects of altering (e.g., mutating) monomers. In addition, the representations can be used to compare polymers and thereby identify important structural and functional characteristics of polymers.
Owner:PORTLAND BIOSCI
Biodegradable collagen-based cornea substitute and preparation method thereof
InactiveCN109125808AHigh homologyHigh purityPharmaceutical delivery mechanismBiochemical treatment with enzymes/microorganismsChemistryCorneal corpuscle
The invention discloses a biodegradable collagen-based cornea substitute and a preparation method thereof. The substitute is obtained as follows: collagen and a macromolecular polymer are subjected tocompound crosslinking treatment in a aqueous phase and then cured by a mol; or the collagen and the macromolecular polymer are subjected to electrospinning to form a nanofiber membrane, the nanofibermembrane is further subjected to compound crosslinking and drying to form a 3D cornea scaffold, and the 3D cornea scaffold is used after rehydration swelling clinically. The cornea substitute has controllable degradability, good phototropism and mechanism strength and significant induction functions on increase of corneal epithelial cells and corneal corpuscles.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
Compound yolk antibody capable of resisting fowl bacterial blight and its preparation and use
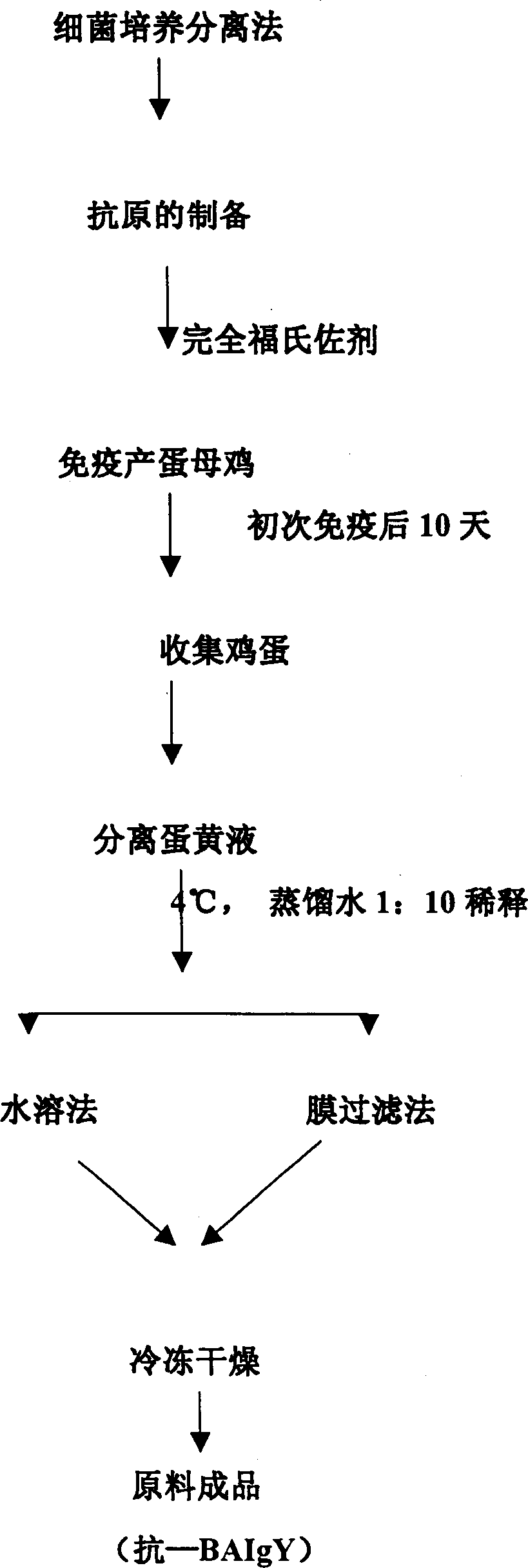
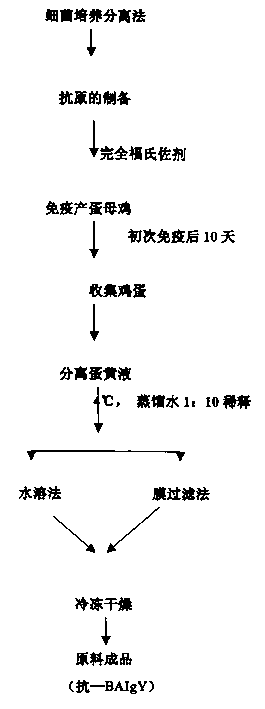
The present invention relates to a compound vitelline antibody (anti-BAIgY) with antibody activity for resisting salmonella gallinarum, bacterium multocidum and chicken colibacillus, method for preparing said antibody and application of said antibody in preparation of medicine or feed additive for curing relatied diseases which can be infected by above-mentioned bacteria. The preparation method of said compound vitelline antibody includes the following steps: preparing compound antigen of the above-mentioned bacteria, immunizing health hen with said antigen, collecting egg and extracting anti-BA IgY from egg.
Owner:重庆和润实业(集团)有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com