Novel coronavirus antigen colloidal gold diagnostic kit
A diagnostic kit, coronavirus technology, applied in the directions of viruses/phages, viruses, viral peptides, etc., can solve the problem of the lack of binding force of the new coronavirus, and achieve the effect of effective control of the epidemic, simple and fast test operation, and accurate diagnosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] This embodiment provides a SARS-COV2 spike protein antibody, which is a paired antibody to the S1 region of the SARS-COV2 spike protein, prepared by the following method:
[0043] 1. Expression and purification of SARS-COV2 spike protein
[0044] (1) fully synthesize the gene encoding the Gln14-Arg685 region of the S1 protein;
[0045](2) The S1 gene was cloned between the IL2 secretion signal peptide of the eukaryotic expression vector pMFcIg (ABLINK biotech) and the mouse Fc (mFc, including hinge-CH2-CH3) tag gene to form the S1-mFc fusion gene, and the cloned The forward primer used is: CAGTGTGTTAATCTTACAACC, and the reverse primer is: ACGTGCCCGCCGAGGAGAATT;
[0046] (3) Transfer the S1-mFc expression plasmid to the competent cell Rosseta, pick the positive single clone, and extract the plasmid after amplification;
[0047] (4) Use 293Fectin (thermofisher) transfection reagent to transfect S1-mFc expression plasmid into 293F cells, continue to culture in serum-free...
Embodiment 2
[0060] This embodiment provides a SARS-COV2 spike protein antibody colloidal gold diagnostic kit, including the paired antibody to the S1 region of the SARS-COV2 spike protein prepared in Example 1, prepared by the following method:
[0061] (1) Take a 0.01% chloroauric acid aqueous solution and heat it to boiling, add dropwise a volume concentration of 1% trisodium citrate solution and keep stirring, and continue to boil for 15 minutes after the chloroauric acid aqueous solution changes from golden yellow to purple. , after cooling, restore to the original volume with distilled water, and store at 2°C to 8°C for later use;
[0062] (2) Concentrate the paired antibody solution of the S1 region of the SARS-COV2 spike protein to 1 mg / mL, transfer it into a dialysis bag, and dialyze it with a Tris buffer with a concentration of 20 mmol / L and a pH value of 7.0 at 2°C to 8°C overnight;
[0063] (3) Add an equal volume of colloidal gold to the paired antibody of the S1 region of th...
Embodiment 3
[0065] The SARS-COV2 spike protein antibody colloidal gold prepared by the method of Example 2 is clinically verified.
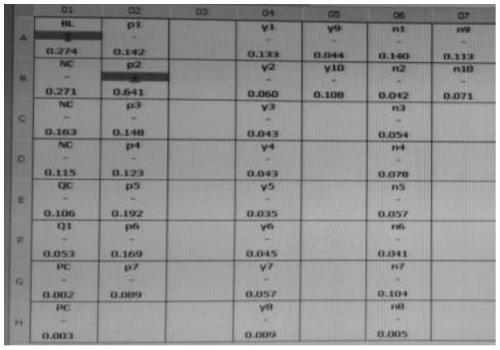
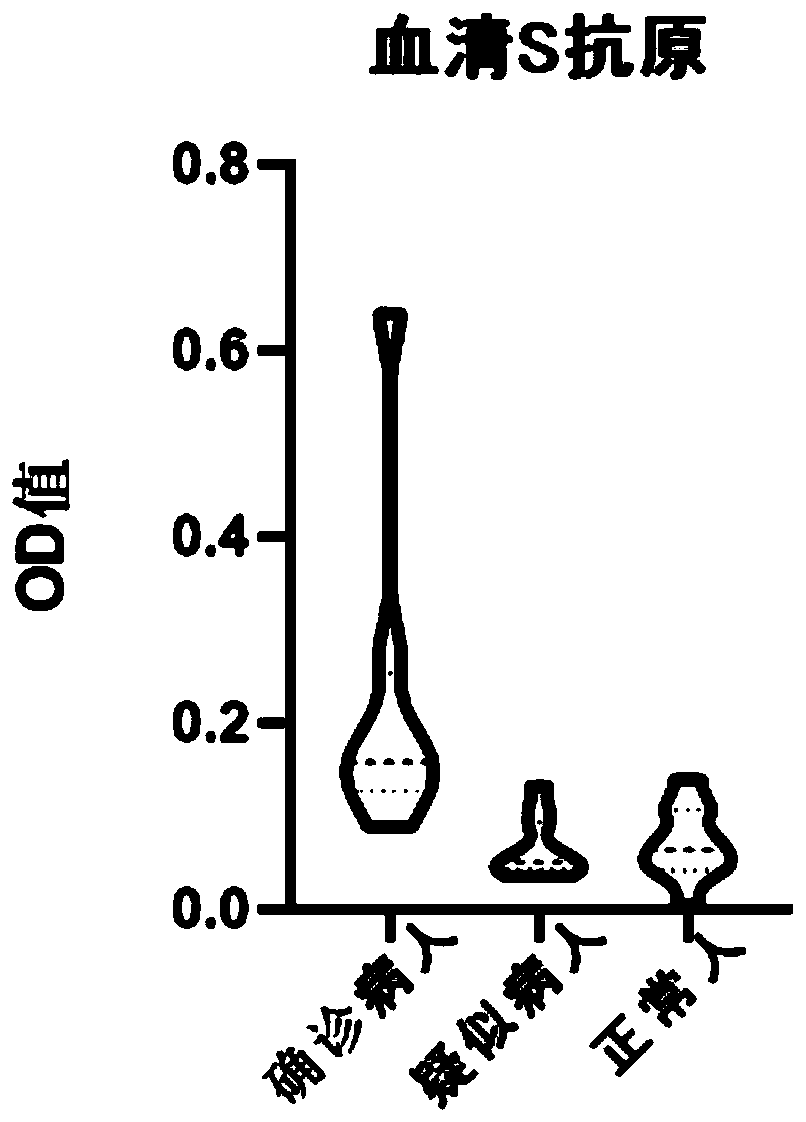
[0066] Take 7 clinically confirmed cases of new coronavirus pneumonia, 10 suspected samples and 10 negative samples for verification. The verification method is as follows:
[0067] Add SARS-COV2 spike protein antibody colloidal gold to a 96-well plate, add 100 μL of the above-mentioned different types of specimens, incubate at 37°C for 1 hour, wash the plate, add primary antibody against another S antigen epitope, and incubate at 37°C for 1 hour Finally, wash the plate, then add the enzyme-labeled secondary antibody against the Fc segment of human IgG, and develop the color with the chromogenic reagent, and the color development result is as follows: figure 1 , utilize a microplate reader to measure the OD value, the results are shown in Table 3, and figure 2 and image 3 Shown; Among them, Table 3 gives the figure 1 The order of adding samples and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com