ELISA kit for distinctively detecting antibodies of classical swine fever (CSF) vaccine immunity and wild virus infection and preparation method thereof
A swine fever vaccine and kit technology, applied in measuring devices, instruments, scientific instruments, etc., can solve problems such as difficulty in ensuring accuracy, difficulty in collection, difficulty in large-scale promotion and application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
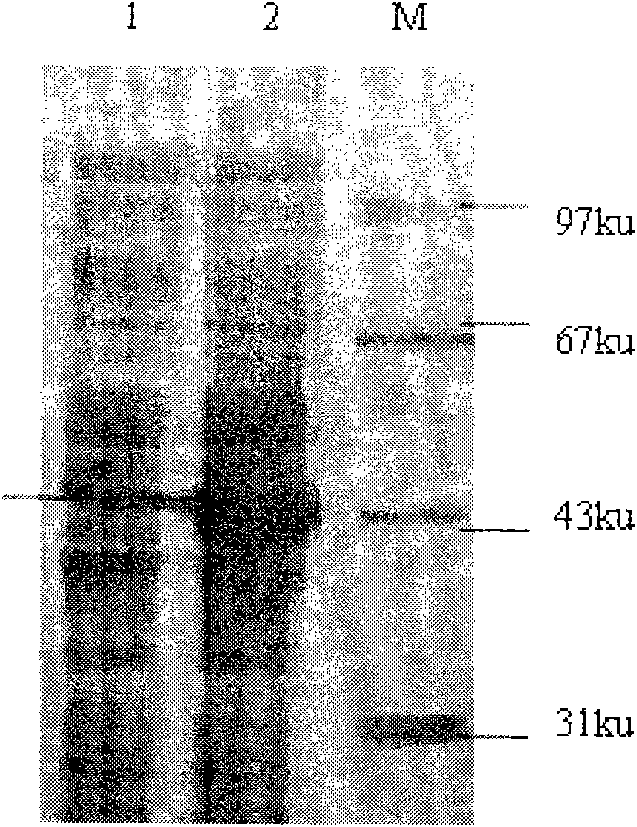
[0087] Example 1 Prokaryotic expression and purification of NS3
[0088]1. Viral RNA extraction: extract the total RNA of hog fever rabbitization attenuated cell culture fluid with TRIzol Reagent kit (purchased from Invitrogen);
[0089] 2. Design and synthesis of primers: Design a pair of primers based on the NS3 gene sequence published in GenBank, and add Sac I and Xho I restriction sites at both ends of the primers respectively, to amplify the 2049 bp gene between 5142 and 7191 bp of the open reading frame fragment. The primers (sequence 1 and sequence 2) sequences are respectively:
[0090] P1: 5' GAG CTC GGG CCT GCC GTT TGC AA-3';
[0091] P2: 5' CTC GAG CTA GAC CAA CTA CCT GTT TTA GTG CTC TG-3';
[0092] The underlined part is the restriction site introduced, and the primers were synthesized by Invitrogen.
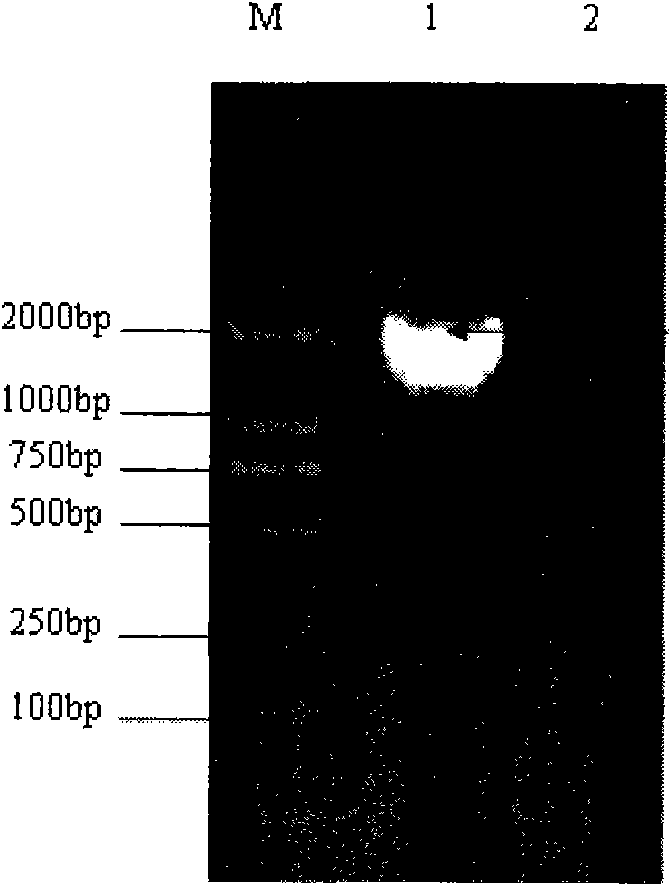
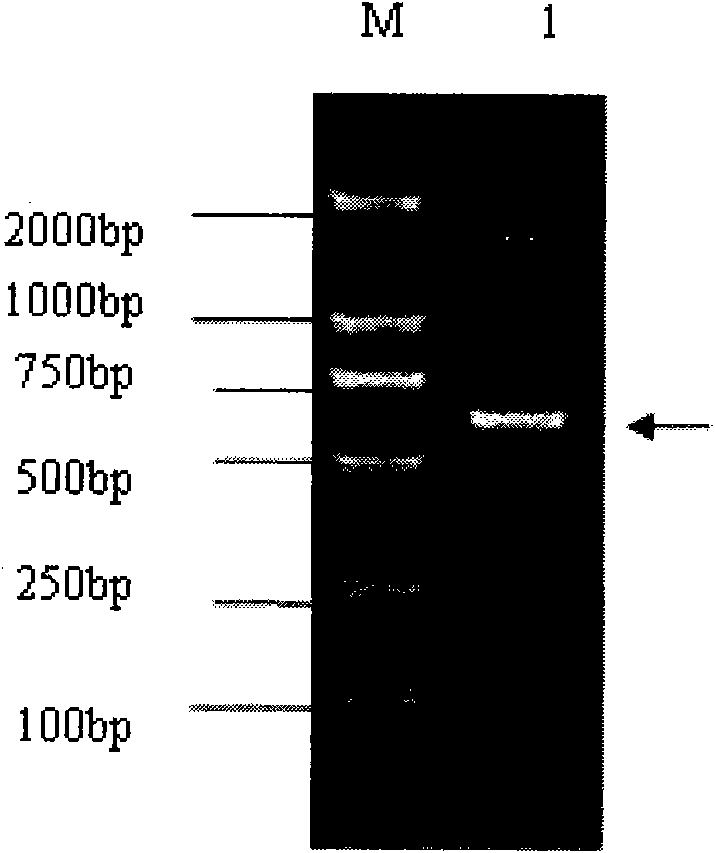
[0093] 3. Amplification and recovery of NS3 gene fragments
[0094] (1) RT-PCR amplification: use the extracted RNA as a template, and use the primers in ...
Embodiment 2
[0105] Example 2 Eukaryotic expression and purification of NS3
[0106] 1. Viral RNA extraction: extract the total RNA of hog fever rabbitization attenuated cell culture fluid with TRIzol Reagent kit (purchased from Invitrogen);
[0107] 2. Primer design and synthesis: Design a pair of primers based on the NS3 gene sequence published by GenBank, and add EcoR I and Xho I restriction sites at both ends of the primers, which can amplify the gene of 2049 bp between the open reading frame 5142 and 7191 bp fragment. The sequences of the primers (SEQ ID NO: 3 and SEQ ID NO: 4) are:
[0108] P3: 5'- GAA TTC GGG CCT GCC GTT TGC AAG-3';
[0109] P4: 5'- CTC GAG CTA GAC CAA CTA CCT GTT TTA GTG CTC-3';
[0110] The underlined part is the restriction site introduced, and the primers were synthesized by Invitrogen.
[0111] 3. Amplification and recovery of NS3 gene fragments
[0112] (1) RT-PCR amplification: use the extracted RNA as a template, and use the primers in step 2 for ...
Embodiment 3
[0126] Example 3 NS3 Antibody Detection Negative and Positive Control Serum Preparation and Reagent Preparation
[0127] 1. Preparation of negative and positive control serum for NS3 antibody detection
[0128] (1) Positive control serum for NS3 antibody detection of swine fever vaccine: Collect blood and separate serum every week after healthy non-immune pigs are immunized with swine fever rabbit attenuated vaccine, collect 10 times in total, combine and mix well as swine fever vaccine immunization NS3 antibody detection Positive control serum.
[0129] (2) Positive control serum for NS3 antibody detection of CSF wild virus infection: After the healthy non-immune pigs were inoculated with low-virulence and moderate-virulence CSFV, blood was collected every week to separate the serum, and the serum was collected 10 times in total, combined and mixed as CSF Positive control serum for wild virus infection NS3 antibody detection.
[0130] (3) Negative control serum for CSF vacc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com