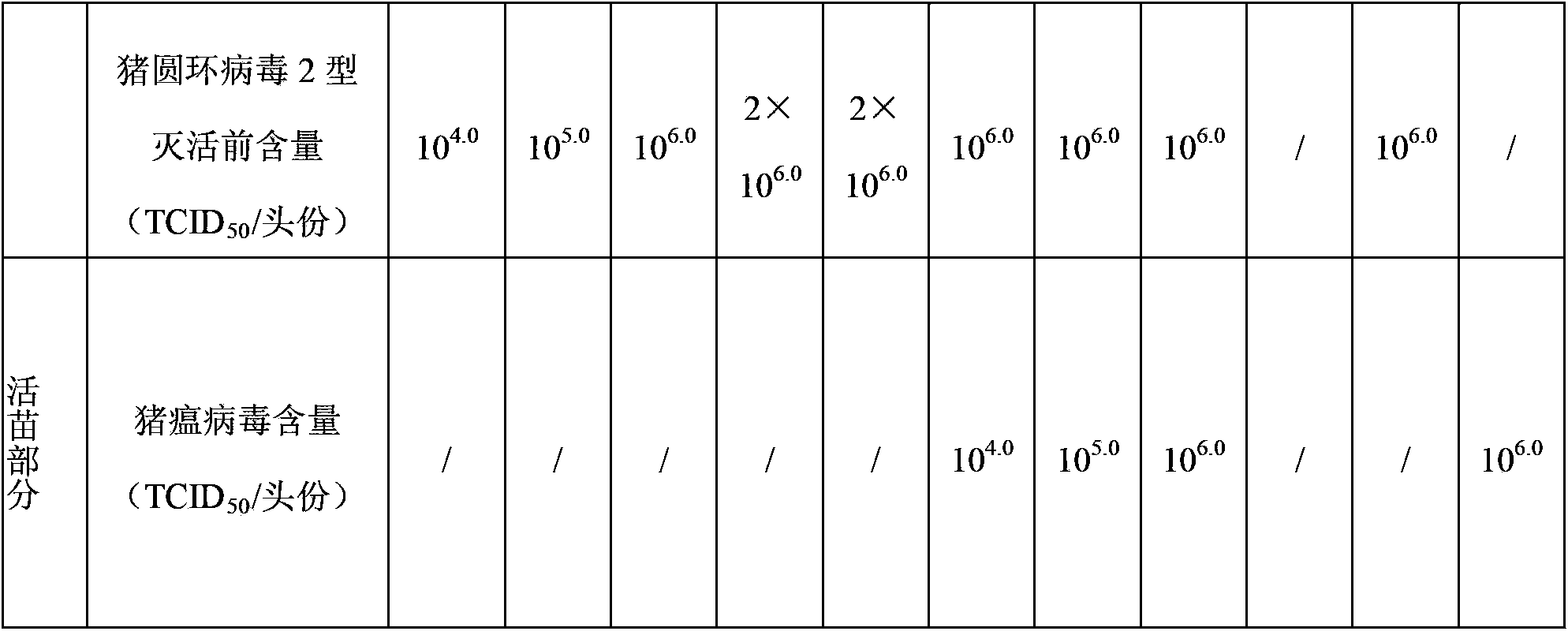Vaccine composition, preparation method and application thereof
A vaccine composition and the technology of the composition are applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, bacterial antigen components, etc., which can solve problems such as mixed infections and economic losses, and achieve avoidance of adverse reactions, reduction of vaccination times, immunity Convenient and fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
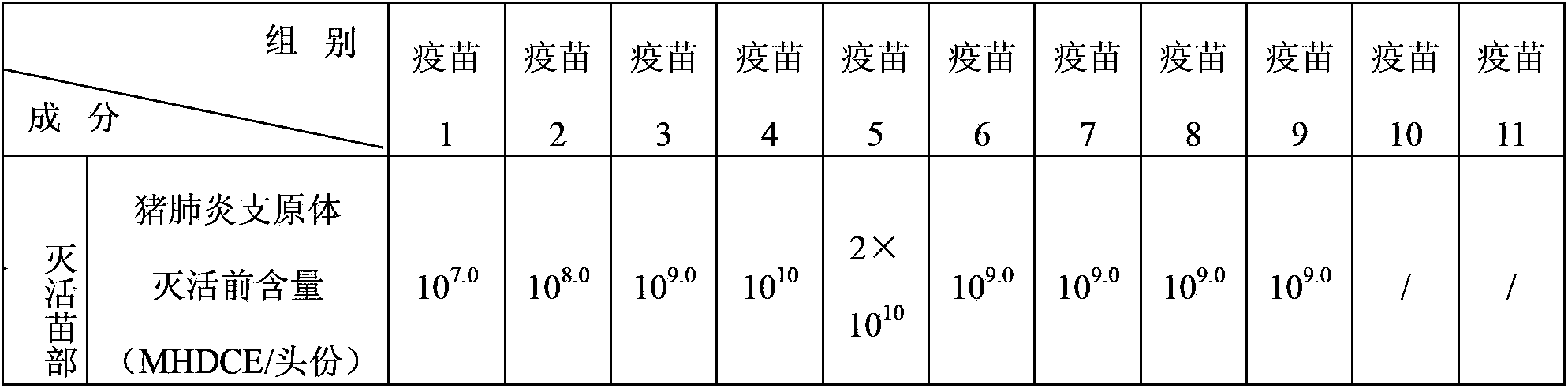
[0030] The preparation of embodiment 1 porcine circovirus type 2 antigen and mycoplasma hyopneumoniae antigen
[0031] 1 strain source
[0032] The type 2 strain of porcine circovirus used in the manufacture and inspection of this product is the PCV-2 strain SH strain, which was preserved in the General Microbiology Center of the China Committee for the Collection of Microorganisms. The preservation date: March 4, 2008, and the preservation number It is CGMCC No.2389.
[0033] The strain of Mycoplasma hyopneumoniae used in the manufacture and inspection of this product is HN0613, which was preserved in the China Center for Type Culture Collection (abbreviation: CCTCC; address: Wuhan University, No. 16, Luojiashan Road, Wuchang District, Wuhan City, Hubei Province), preservation date: 2012 June 13, 2012, deposit number: CCTCC NO.M2012230.
[0034] The classical swine fever virus strain used in the manufacture and inspection of this product is the attenuated strain of classic...
Embodiment 2
[0055] The preparation of embodiment 2 swine fever, porcine circular ring and mycoplasma hyopneumoniae triple mixed vaccine composition
[0056] 1 Preparation of porcine circular and mycoplasma hyopneumoniae dual inactivated vaccine
[0057] 1.1 Preparation of preservatives
[0058] 1% (w / v) thimerosal aqueous solution: 1g of thimerosal is dissolved in 100ml of purified water, and autoclaved at 121°C for 30 minutes for later use.
[0059] 1.2 Preparation of diluent
[0060] Sterile PBS buffer solution: Dissolve 8g sodium chloride, 0.25g potassium chloride, 3.63g disodium hydrogen phosphate, 0.24g potassium dihydrogen phosphate in 900ml purified water, then dilute to 1L, autoclave at 121°C for 30min spare.
[0061] 1.3 Vaccine adjuvant treatment
[0062] Sterilization of Gel01 (SEPPIC, France) adjuvant: transfer Gel01 (SEPPIC, France) adjuvant into a sterilizable container, and autoclave at 121°C for 30 minutes for later use.
[0063] 1.4 Matching seedlings
[0064] Thr...
Embodiment 3
[0072] The swine fever of embodiment 3 different antigen contents, porcine circular ring and mycoplasma hyopneumoniae triple combination vaccine effectiveness test
[0073] 1 test material
[0074] Vaccine 1 (mycoplasma hyopneumoniae 10) prepared in embodiment 2 7 MHDCE / Toufen, porcine circovirus type 2 10 4.0 TCID 50 / head), vaccine 2 (Mycoplasma hyopneumoniae 10 8 MHDCE / Toufen, porcine circovirus type 2 10 5.0 TCID 50 / head), vaccine 3 (Mycoplasma hyopneumoniae 10 9 MHDCE / Toufen, porcine circovirus type 2 10 6.0 TCID 50 / head), vaccine 4 (Mycoplasma hyopneumoniae 10 10 MHDCE / Toufen, porcine circovirus type 2 2×10 6.0 TCID 50 per serving) and vaccine 5 (Mycoplasma hyopneumoniae 2×10 10 MHDCE / Toufen, porcine circovirus type 2 2×10 6.0 TCID 50 / toufen).
[0075] 3 to 4 weeks old, weaned piglets without antibodies to CSF, Circular swine and Mycoplasma hyopneumoniae.
[0076] 2 test method
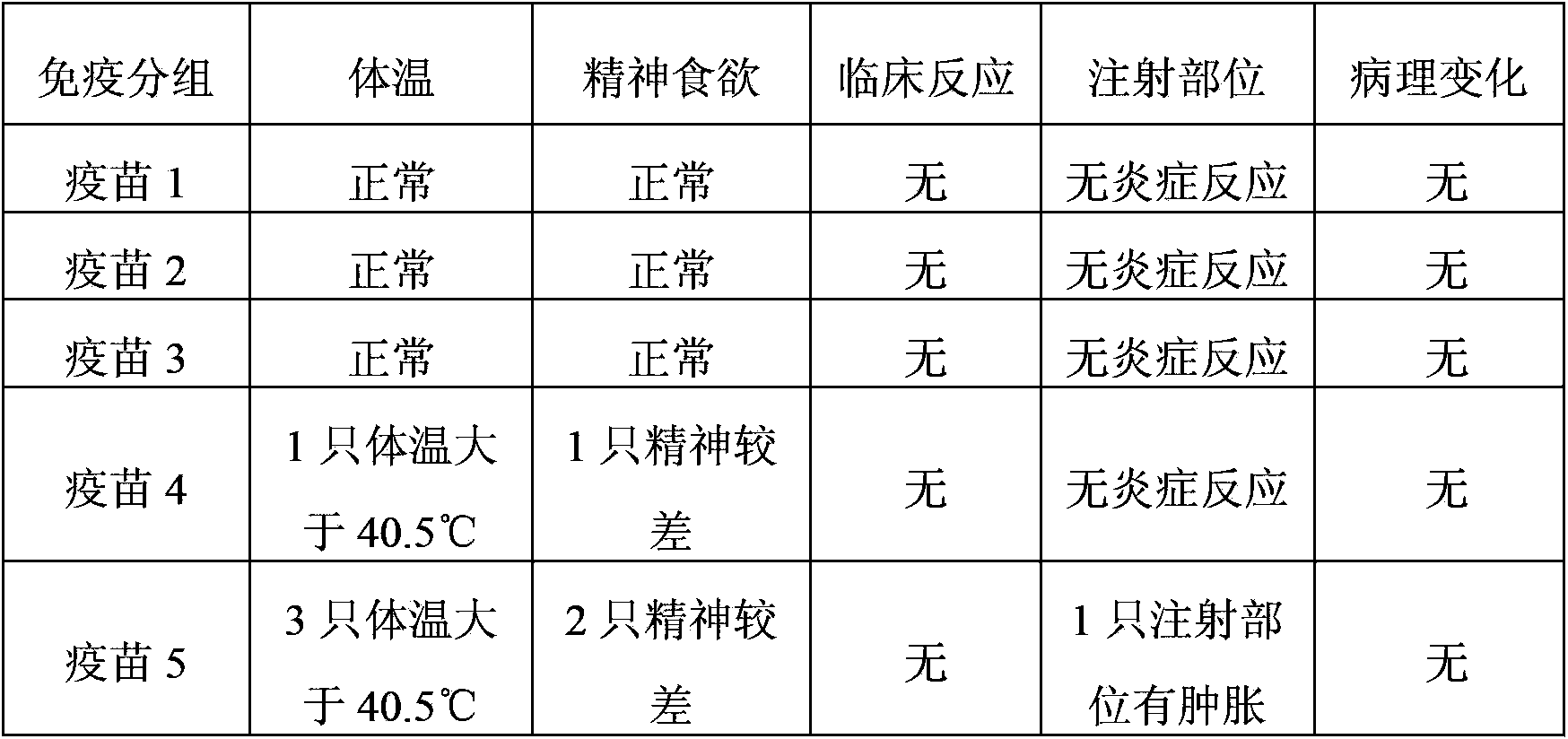
[0077] 2.1 Safety test
[0078] Select 30 weaned piglets aged 3 to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com