Haemophilus parasuis disease vaccine composition, preparation method and application thereof
A technology for Haemophilus suis disease and vaccine composition, which is applied in the directions of drug combination, antibacterial drugs, respiratory system diseases, etc., can solve the problems that vaccines are difficult to achieve immune effect, interference, etc., and achieve effective treatment and prevention of haemophilus parasuis Bacillus related diseases, unsatisfactory solution effect, good immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
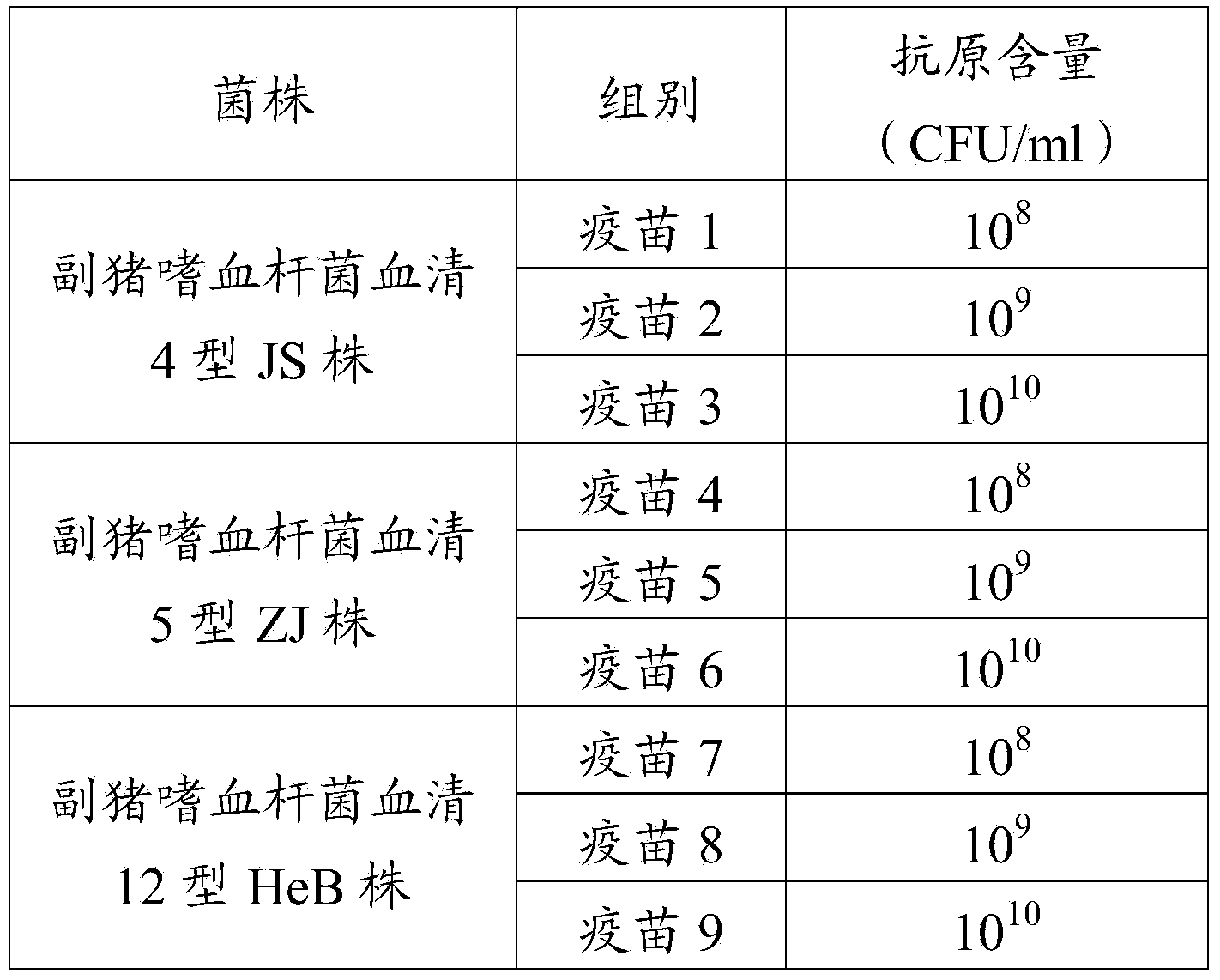
[0038] Preparation and efficacy test of embodiment 1 Haemophilus parasuis inactivated vaccine
[0039] 1. Strains
[0040] a. Haemophilus parasuis serotype 4 JS strain, taxonomic name: Haemophilus parasuis JS (Haemophilus parasuis JS), isolated and identified by Pulaike Bioengineering Co., Ltd., has been carried out in the China Center for Typical Cultures Deposit, date of deposit: May 18, 2011, deposit number: CCTCC M2011172.
[0041] b. Haemophilus parasuis serotype 5 ZJ strain, classified and named: Haemophilus parasuis ZJ (Haemophilus parasuis ZJ), isolated and identified by Pulaike Bioengineering Co., Ltd., and has been carried out in the China Center for Typical Cultures Deposit, date of deposit: May 18, 2011, deposit number: CCTCC M2011173.
[0042] c. HeB strain of Haemophilus parasuis serotype 12, classified and named: Haemophilus parasuis HeB (Haemophilus parasuis HeB), which was isolated and identified by Pulaike Bioengineering Co., Ltd., and has been carried out ...
Embodiment 2
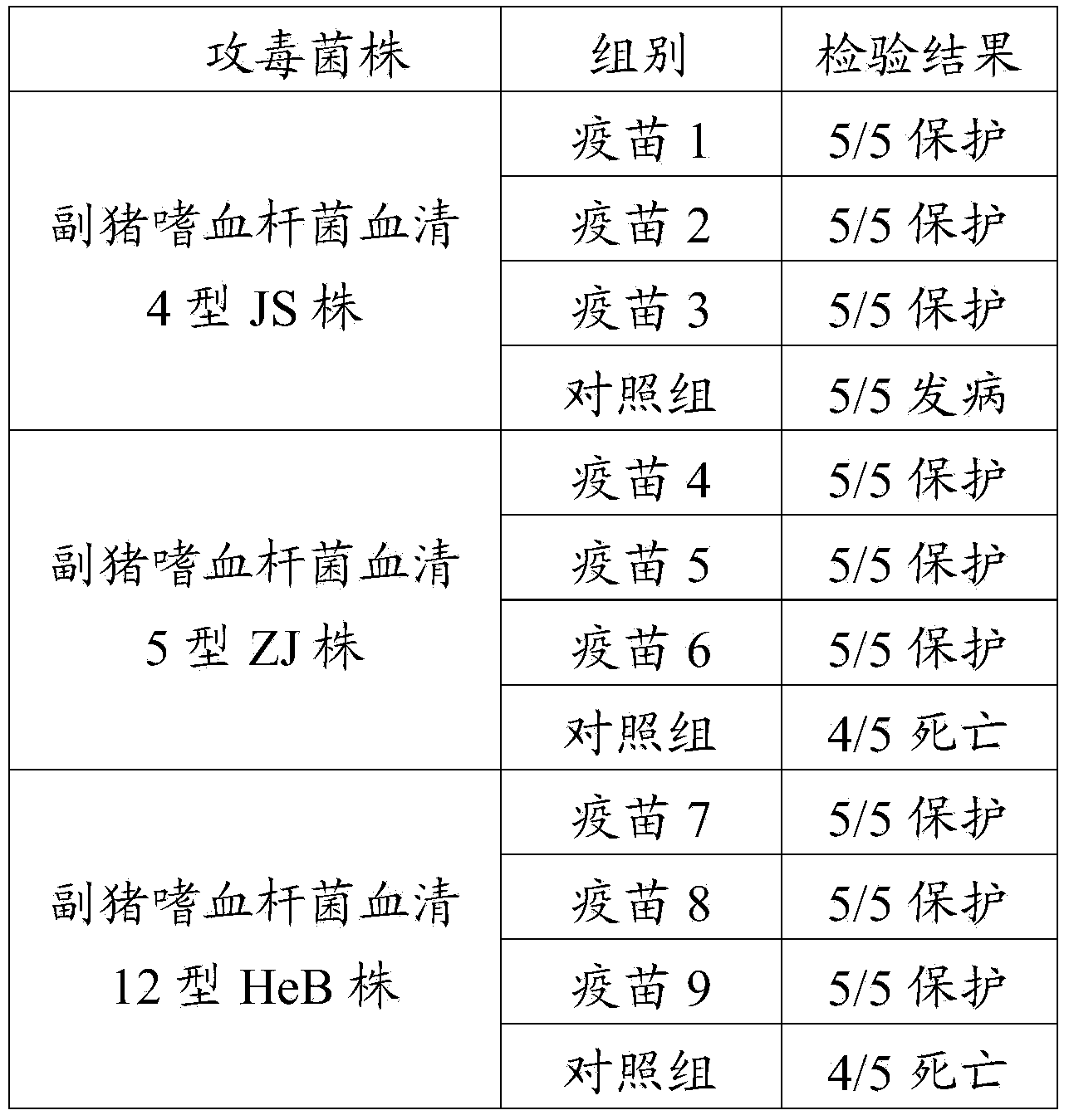
[0079] Embodiment 2 cross-protection test
[0080] Experiments were carried out in the experimental animal room of Pulaike Bioengineering Co., Ltd. Take the vaccine 2, vaccine 5 and vaccine 8 prepared above, and each group of vaccines uses 15 28-day-old healthy susceptible pigs (raised and provided by the experimental animal room of Pulaike Bioengineering Co., Ltd.), and injects 2 mL each intramuscularly. Containing one dose, after 28 days, the immune test pigs injected with each group of vaccines were randomly divided into three groups, and challenged with the serotype 4, 5 and 12 strains of Haemophilus parasuis respectively. At the same time, 15 control pigs were selected, divided into three groups, and challenged with the H. parasuis serotype 4, 5, and 12 strains respectively. details as follows:
[0081] Select 15 pigs for the immunization test of Haemophilus parasuis vaccine 2 prepared in Example 1, divide them into three groups at random, and inject 1 morbidity dose of...
Embodiment 3
[0089] The novel inactivated vaccine of Haemophilus parasuis disease prepared by different adjuvants and efficacy test of embodiment 3
[0090] Utilize different adjuvants to prepare Haemophilus parasuis inactivated vaccines: prepared from the Haemophilus parasuis serotype 4 strains prepared in Example 1 that have been removed from the capsule, inactivated, concentrated and passed the safety inspection, ready to use Antigens were prepared from Haemophilus parasuis serotype 4 strains and mixed with Montanide IMS1313VG, Montanide IMS251C VG, Montanide ISA15A VG, Montanide GEL PR or aluminum hydroxide gel adjuvants to prepare vaccines. The specific formula is shown in Table 4.
[0091] Preparation of aqueous adjuvant vaccine: inactivated Haemophilus parasuis vaccine made with Montanide IMS1313VG or Montanide IMS251C VG or Montanide GEL PR as adjuvant. The specific method is as follows: Concentrate the bacterial solution with a hollow fiber ultrafilter, discard the supernatant, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com