Method for preparing triple inactivated vaccine
An inactivated vaccine and inactivated technology, applied in the field of vaccines, can solve the problems of high cost, mixed infection, and high stress in pigs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 1
[0021] Embodiment 1 A kind of preparation method of triple inactivated vaccine
[0022] A kind of preparation method of haemophilus parasuis disease, streptococcus suis, mycoplasma swine pneumonia triple inactivated vaccine, its steps are as follows:
[0023] 1 Production strain preparation
[0024] 1.1 First-class seed propagation
[0025] Streak-inoculate the freeze-dried strains of Haemophilus parasuis type 4, Haemophilus parasuis type 5, and Streptococcus suis type 2 on TSA / NAD plates, culture at 37°C for 18-24 hours, and pick a single colony Inoculate in TSB / NAD liquid medium, culture at 37°C for 12-16 hours, it is a first-class seed; freeze-dried strains of Mycoplasma hyopneumoniae are streaked and inoculated on solid medium, cultured at 37°C for 7 days, pick a single colony for inoculation On the slant solid medium, cultured at 37°C for 7 days, it is the first-class seed.
[0026] 1.2 Secondary seed propagation
[0027]The first-grade seeds of Haemophilus parasuis t...
Embodiment 2
[0051] Embodiment 2: The safety research of the vaccine prepared in embodiment 1
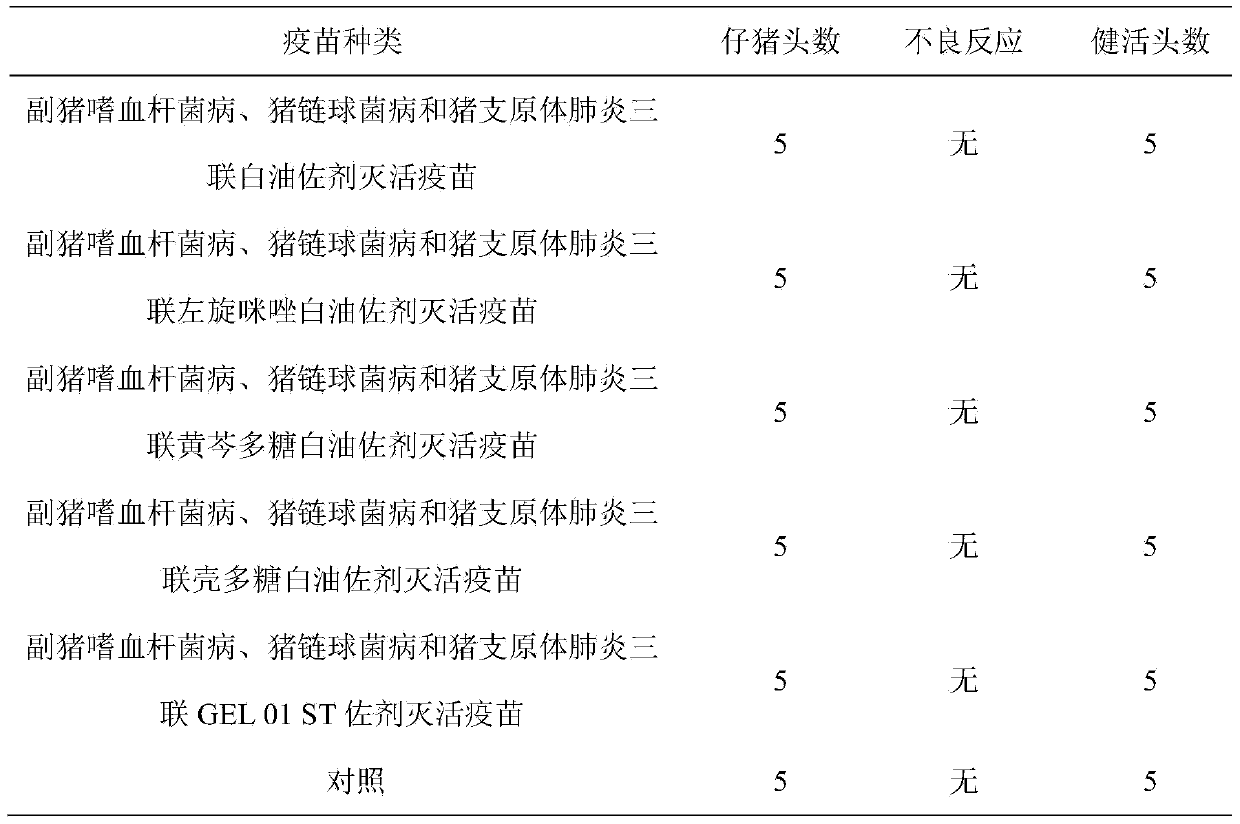
[0052] Get the vaccine prepared in Example 1 and inject intramuscularly into healthy susceptible weaned piglets at the age of 4 to 5 weeks, 2 mL per head (including 1 head), inject 5 heads of each vaccine, and 5 heads of the control group. After 2 weeks, use the same Methods and the same dose of the second immunization, observed for 2 weeks, there should be no local or systemic adverse reactions and all healthy and alive. The results are shown in Table 1.
[0053] Table 1 Safety study results
[0054]
[0055] Safety test results show: Haemophilus parasuis, Streptococcus suis and Mycoplasma pneumonia triple white oil adjuvant inactivated vaccine, Haemophilus parasuis, Streptococcus suis and Mycoplasma pneumonia triple levamisole white oil Adjuvanted Inactivated Vaccine, Haemophilus Parasuis, Streptococcus Suis and Mycoplasma Pneumonia Triple Scutellaria Polysaccharide White Oil Adjuvanted I...
Embodiment 3
[0056] Example 3: Vaccine Efficacy Study
[0057] 1 Experimental design
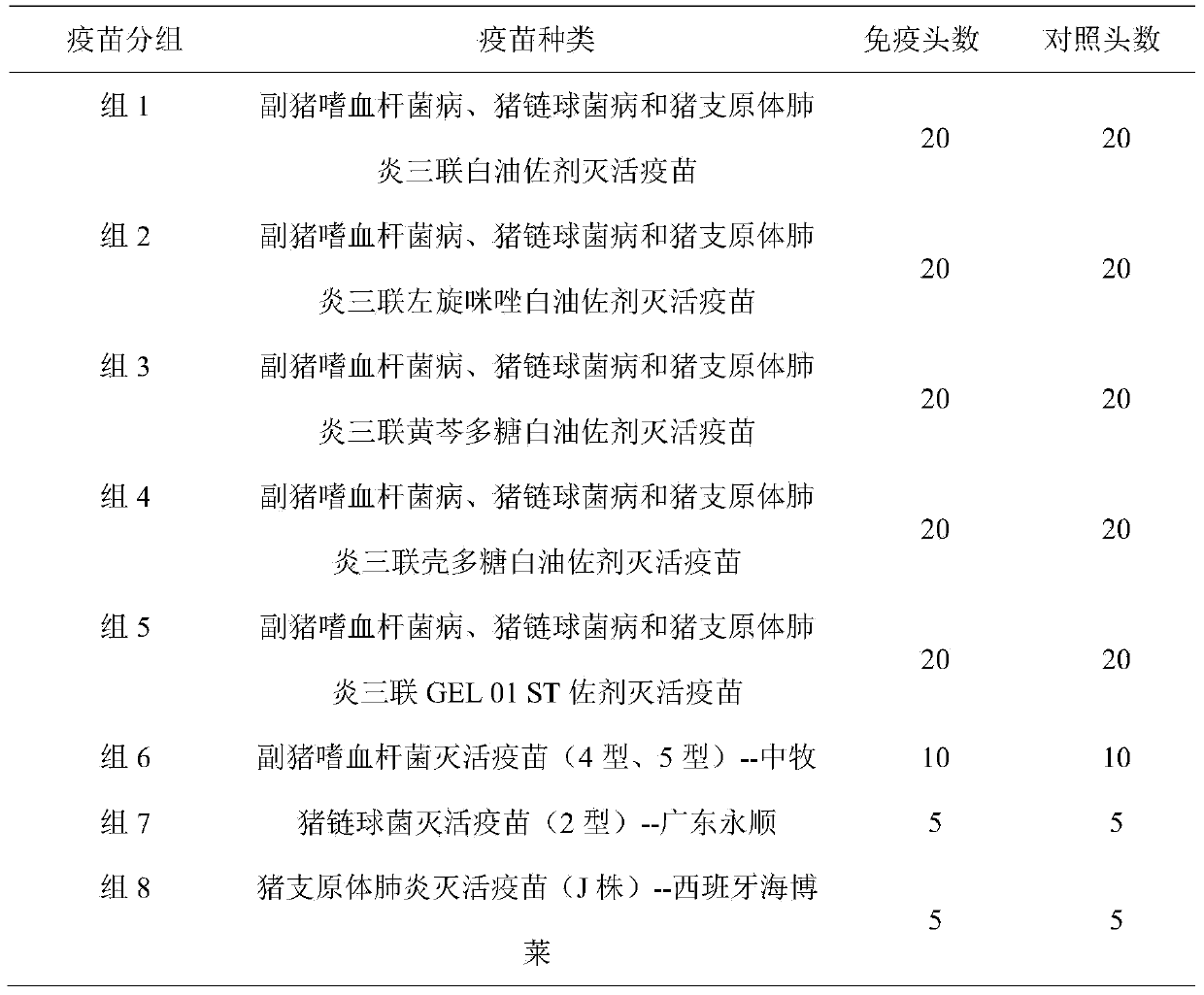
[0058] 1.1 Vaccine Immunization
[0059] Get the vaccine prepared in Example 1, inject various vaccines into 4-5 weeks old healthy susceptible weaned piglets, 2mL per head (one head portion), 20 pigs in each group, the second immunization after the first immunization 3 weeks, the same immunization dose, At the same time, 20 pigs in each vaccine group were used as controls, which were not immunized.
[0060] Purchase commercially available Haemophilus parasuis inactivated vaccines (types 4 and 5), purchased from China Animal Husbandry Co., Ltd., batch number 1209006; Streptococcus suis inactivated vaccines (type 2), purchased from Guangdong Yongshun Biological Products Co., Ltd., batch number 2012010; inactivated vaccine for mycoplasma swine pneumonia (strain J), purchased from Spain's Hyperion Company, batch number 31KB-1. The vaccines purchased above were injected into 4-5 week-old healthy susceptibl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com