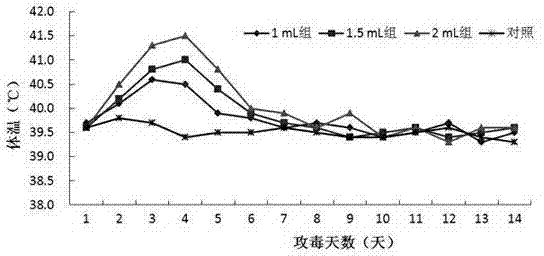
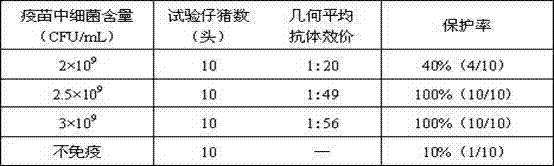
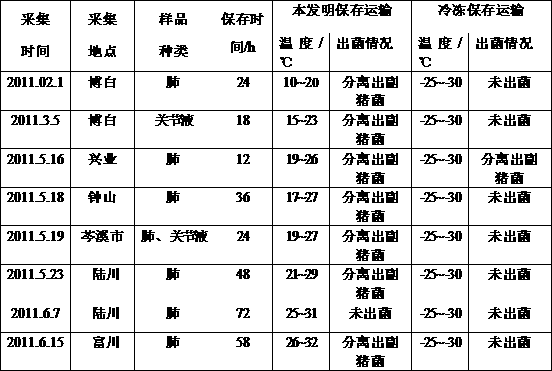
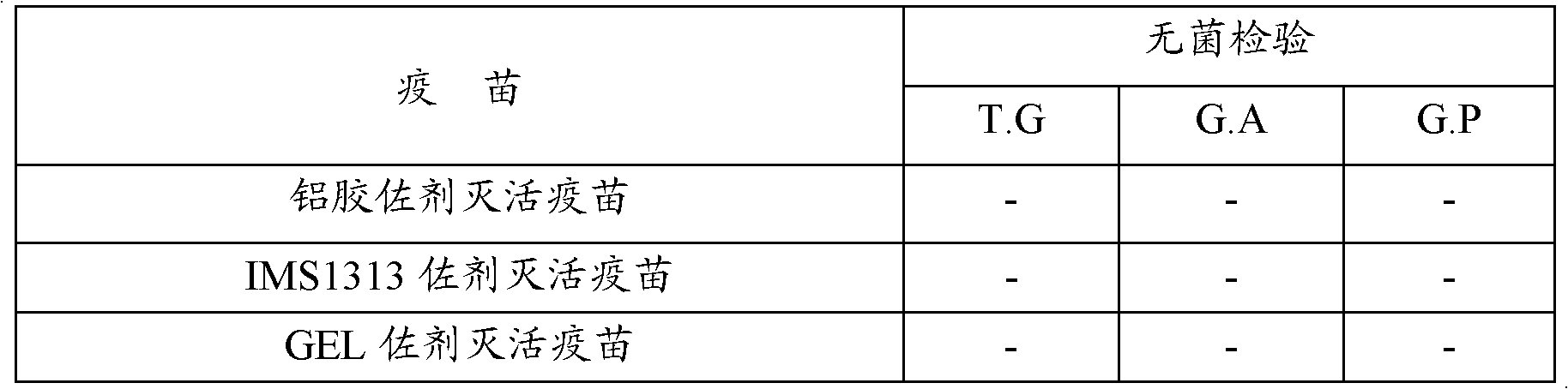
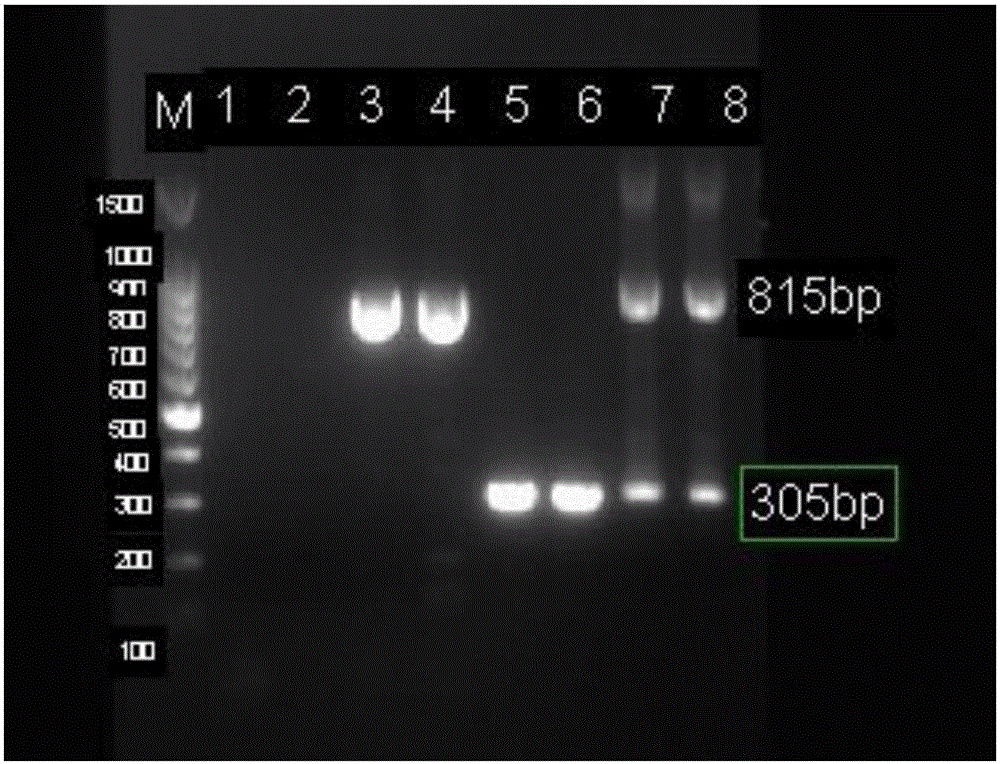
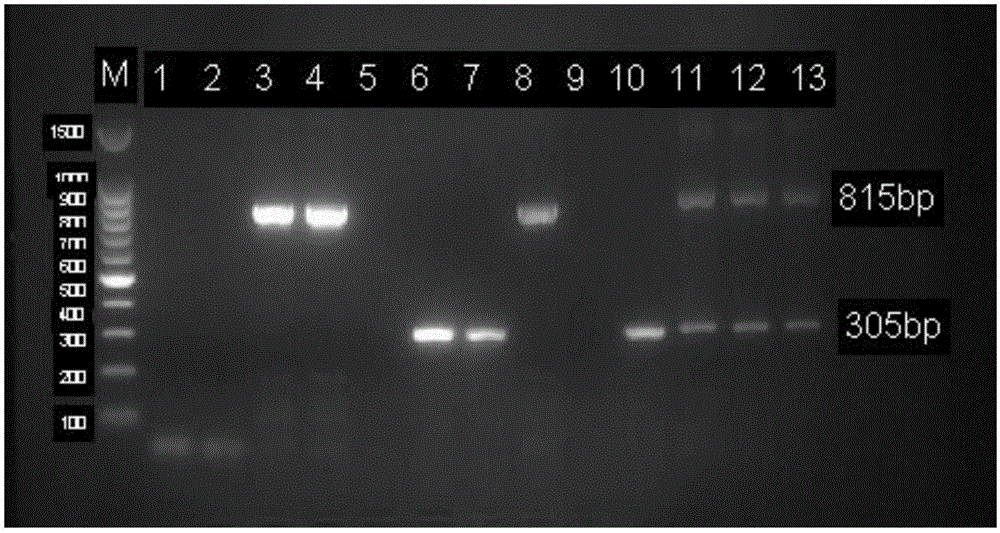
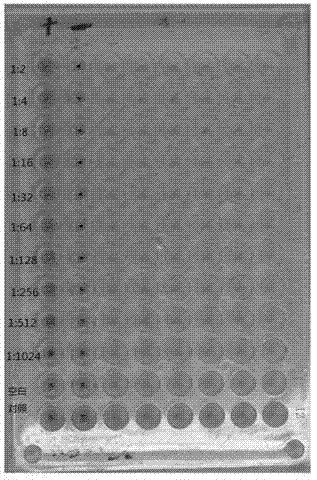
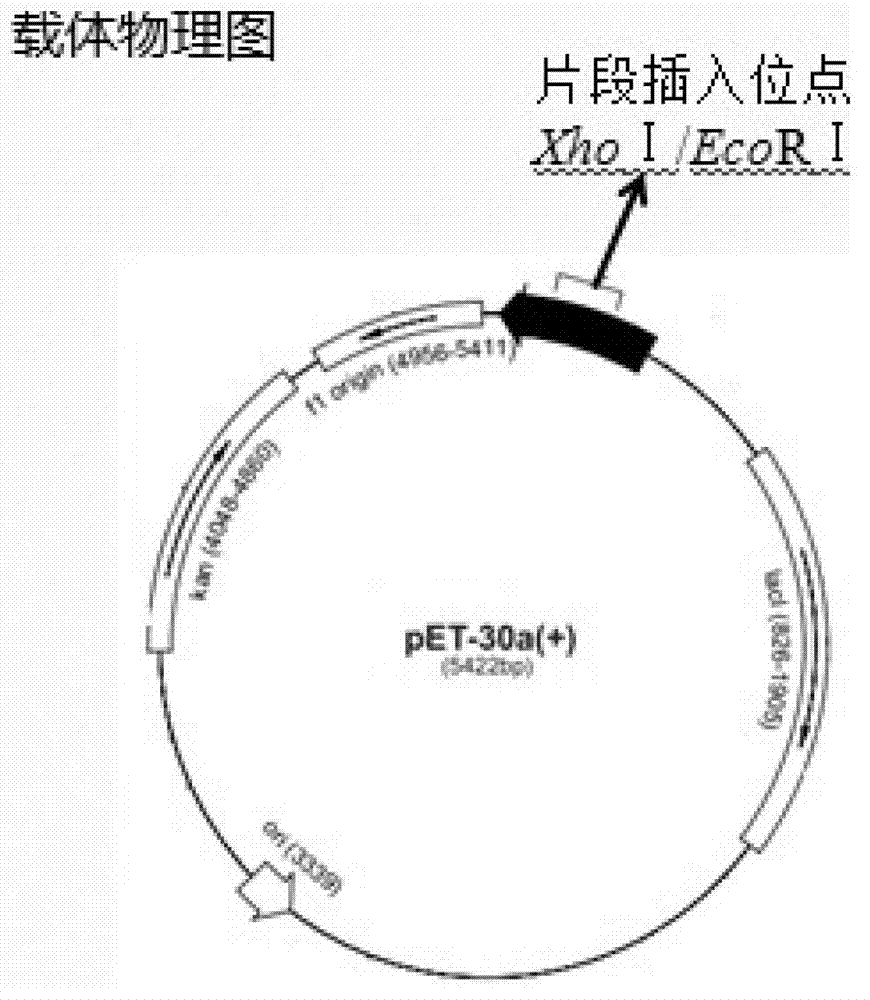
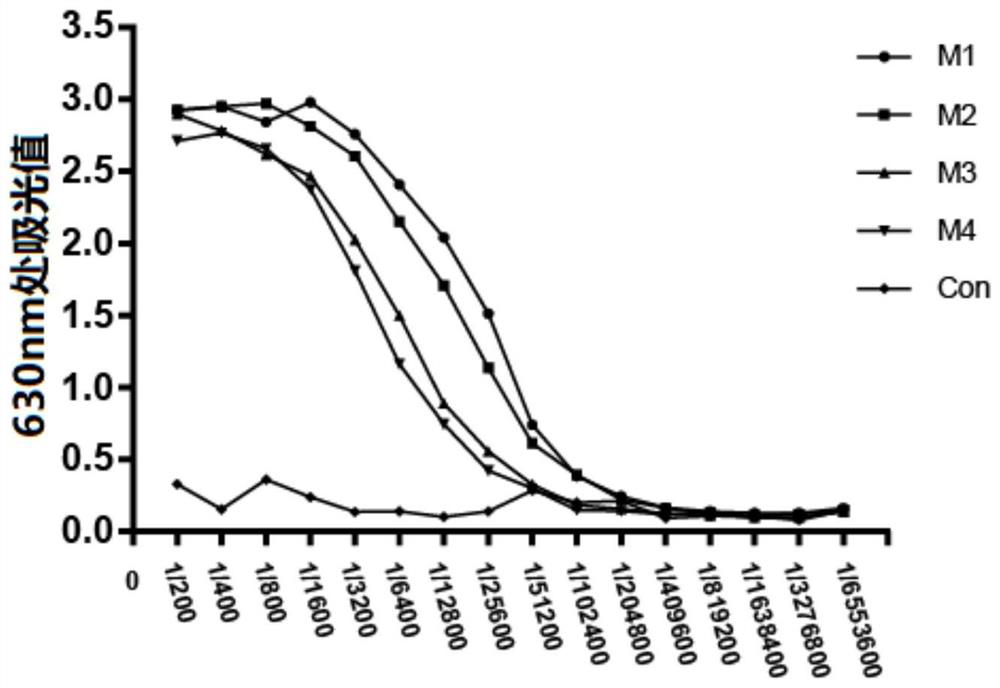
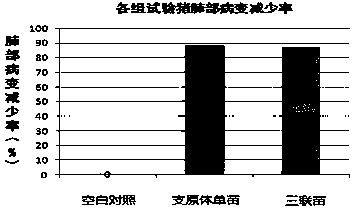
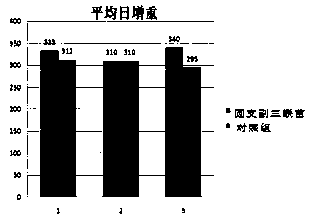
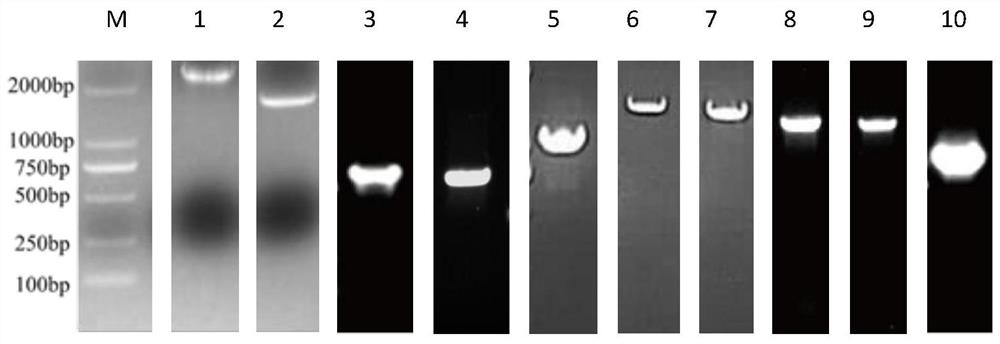
Patents
Literature
115 results about "Hemophilus parasuis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Haemophilus parasuis is a species of the Haemophilus genus. It inhabits the nasopharynx of normal swine. Serotype B of the bacteria causes Glasser's disease in pigs. It can also cause fibrinous polyserositis, polyarthritis and meningitis.
Indirect ELISA (Enzyme-Linked Immunosorbent Assay) method and kit for detecting haemophilus parasuis antibodies
InactiveCN101968490AGood specificityHigh sensitivityBacteria peptidesBiological testingEpidemiologic surveyEnzyme
The invention discloses indirect ELISA (Enzyme-Linked Immunosorbent Assay) method and kit for detecting haemophilus parasuis antibodies. The indirect ELISA method comprises the steps of: taking a haemophilus parasuis OMPP5 protein as an envelope antigen; wherein the judgment standard is as followed: OD450 value of to-be-detected serum is greater than 0.375, OD450 value of the to-be-detected serum / OD450 value of standard negative serum is greater than or equal to 2.1. The kit for realizing the method comprises an envelope buffer solution, confining liquid, a washing buffer solution, an antibody diluent, a chromogenic substrate solution and a stop solution, wherein the envelop antigen used in the method is the haemophilus parasuis OMPP5 protein. Proved by a specificity test, a blocking-up experiment, a repeatability test and clinic application detection, the invention has the characteristics of excellent specificity, high sensitiveness, good repeatability, rapidness, simpleness and accuracy, and can be used in clinic large-scale detection of haemophilus parasuis antibodies and epidemiological survey.
Owner:广东省农业科学院兽医研究所
Haemophilus parasuis LC strain and application thereof
ActiveCN102399724AStrong pathogenicityImproving immunogenicityAntibacterial agentsBacteriaHeterologousDisease
The invention relates to the field of haemophilus parasuis vaccines in veterinary biological products, in particular to a haemophilus parasuis LC strain. The collection number of the strain is CGMCC (China General Microbiological Culture Collection Center) No.5257. The invention also relates to application of the haemophilus parasuis LC strain to preparation of haemophilus parasuis inactivated vaccines. The haemophilus parasuis LC strain has stronger pathogenicity to pigs and has better immunogenicity; an inactivated alumina gel vaccine prepared by the strain is safe and reliable; not only a homologous attacking protection is provided, but also a better cross protection to blood serums type 4, type 5, type 10, type 12, type 14 and type 15 HPS (Hantavirus Pulmonary Syndrome) heterologous attacking can be provided; after the pigs are immunized, a stronger immunity can be generated and the morbidity and the mortality of the inoculated pigs are obviously reduced; the immune effect achieves or is better than the traditional commercialized vaccines in the market; the vaccine has the advantages to compete with like products at home and abroad and is capable of effectively preventing the epidemic and the transmission of a haemophilus parasuis disease and reducing the economic losses caused by the disease, so that the application range is wide.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Haemophilus parasuis culture medium
ActiveCN103215208AMaintain integrityIncrease success rateBacteriaMicroorganism based processesSerum igeHaemophilus
The invention discloses a haemophilus parasuis culture medium. A preparation method of the haemophilus parasuis culture medium comprises the steps of preparing a basic culture solution, treating coenzyme A, and perfecting a culture medium, wherein the basic culture solution comprises peptone, tryptone, sodium chloride, dextrose, yeast extract, glycerin and distilled water, and the basic culture solution, the coenzyme A and fetal bovine serum are uniformly mixed so as to obtain the haemophilus parasuis culture medium. The haemophilus parasuis culture medium provided by the invention can keep haemophilus parasuis, thereby facilitating the transportation of suspicious haemophilus parasuis samples; and the death of haemophilus parasuis is not caused in the process of transportation, thereby increasing the separation probability of the haemophilus parasuis.
Owner:广西悦牧生物科技有限公司
Hemophilus parasuis disease, swine streptococcosis bivalent inactivated vaccine and preparation method thereof
ActiveCN103157100ALittle side effectsFulfil requirementsAntibacterial agentsBacterial antigen ingredientsDiseaseAntigen
The present invention relates to a hemophilus parasuis disease, a swine streptococcosis bivalent inactivated vaccine and a preparation method thereof. The vaccine is prepared by adopting a hemophilus parasuis serotype 4 JS strain, a hemophilus parasuis serotype 5 ZJ strain and a streptococcus suis type 2 SC strain as antigens, and adopting a veterinarily acceptable adjuvant, concurrently provides immunoprophylaxis for hemophilus parasuis diseases caused by hemophilus parasuis, swine streptococcosis caused by streptococcus suis, and mixed infection of the hemophilus parasuis disease and the swine streptococcosis, has effects of low side effect, no endotoxin, no impurity protein in serum, good immunization safety, multi-prevention effect with one needle in the clinic, and cost reducing, and can meet different requirements of different users.
Owner:PU LIKE BIO ENG
Inactivated mixed vaccine for porcine respiratory disease and the method of manufacturing thereof
InactiveCN1942204AIncrease weightIncrease salesSsRNA viruses positive-senseViral antigen ingredientsPost weaningDisease
The present invention relates to inactivated mixed vaccine for preventing porcine respiratory disease. The inactivated mixed vaccine of the present invention, which comprises a certain amount of inactivated Haemophilus parasuis S4 and S5, Mycoplasma hyopneumoniae , and PRRS virus, can effectively prevent porcine respiratory disease such as Glasser's disease, Swine enzootic pneumonia and PRRS. Further, the mixed vaccine of the present invention can prevent progress of the porcine respiratory disease to PRDC (Porcine Respiratory Disease Complex) and PMWS (Post-weaning Multisystemic Wasting Syndrome).
Owner:CHOONG ANG VACCINE LAB
Visualized loop-mediated isothermal amplification kit for detecting Haemophilus parasuis
InactiveCN102776283AStrong specificityIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesAnimal foodPeptidoglycan
The invention discloses a visualized loop-mediated isothermal amplification kit for detecting Haemophilus parasuis. The kit provided by the invention comprises loop-mediated isothermal amplification reaction solution, fluorescent color-developing agent, positive control and negative control, wherein the positive control is Haemophilus parasuis DNA (deoxyribonucleic acid) and the negative control is distilled water. The loop-mediated isothermal amplification reaction solution comprises three pairs of primers, i.e. an inner primer pair, an outer primer pair and a loop primer pair are designed by reference to a conserved region of a Haemophilus parasuis peptidoglycan-associated lipoprotein gene (palA). The kit provided by the invention has the advantages that the detection sensitivity is high, the lowest detection limit of genome DNA is 0.04pg, the kit is simple and convenient to operate, and the kit is particularly suitable for detection of pathogens in fields at a basic level and detection of Haemophilus parasuis in possibly polluted animal food.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Indirect ELISA (enzyme-linked immuno sorbent assay) kit for detecting haemophilus parasuis antibody
ActiveCN103941020AHigh homologyImproved conservatismBiological material analysisBiological testingToxin proteinCytolethal distending toxin
The invention discloses an indirect ELISA (enzyme-linked immuno sorbent assay) kit for detecting a haemophilus parasuis antibody. The kit consists of an ELISA coating plate with haemophilus parasuis cytolethal distending toxin)-C protein serving as a coating antigen, a to-be-detected sample dilution plate, a positive contrast serum, a negative contrast serum, 20-time concentrated washing liquid, a serum sample dilution solution, an enzyme-labeled antibody working solution, a developing solution and a terminating solution. A judgment standard is that if an S / P value is less than 0.200, a sample to be detected is negative; if the S / P value is greater than or equal to 0.200, the to-be-detected sample is positive; the S / P value is obtained according to a formula: S / P value=(the mean value of the to-be-detected sample OD450nm-the mean value of a negative contrast OD450nm) / (the mean value of a positive sample OD450nm-the mean value of the negative contrast OD450nm). Due to the specificity test, the sensitivity test, the repetitiveness test, the coincidence rate test, the test for comparing the kit disclosed by the invention with a kit on sale, the clinical application test and the like, the kit disclosed by the invention has the characteristics of high specificity, high sensitivity, high repetitiveness and the like and is high in coincidence rate to the same type of products on sale home and abroad; the indirect ELISA kit can be used for clinical large-scale detection and epidemiological investigation for the haemophilus parasuis antibodies.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Haemophilus parasuis trivalent inactivated vaccine as well as production method and application thereof
InactiveCN108441446AImprove securityImprove immune efficiencyAntibacterial agentsBacterial antigen ingredientsDiseaseHaemophilus
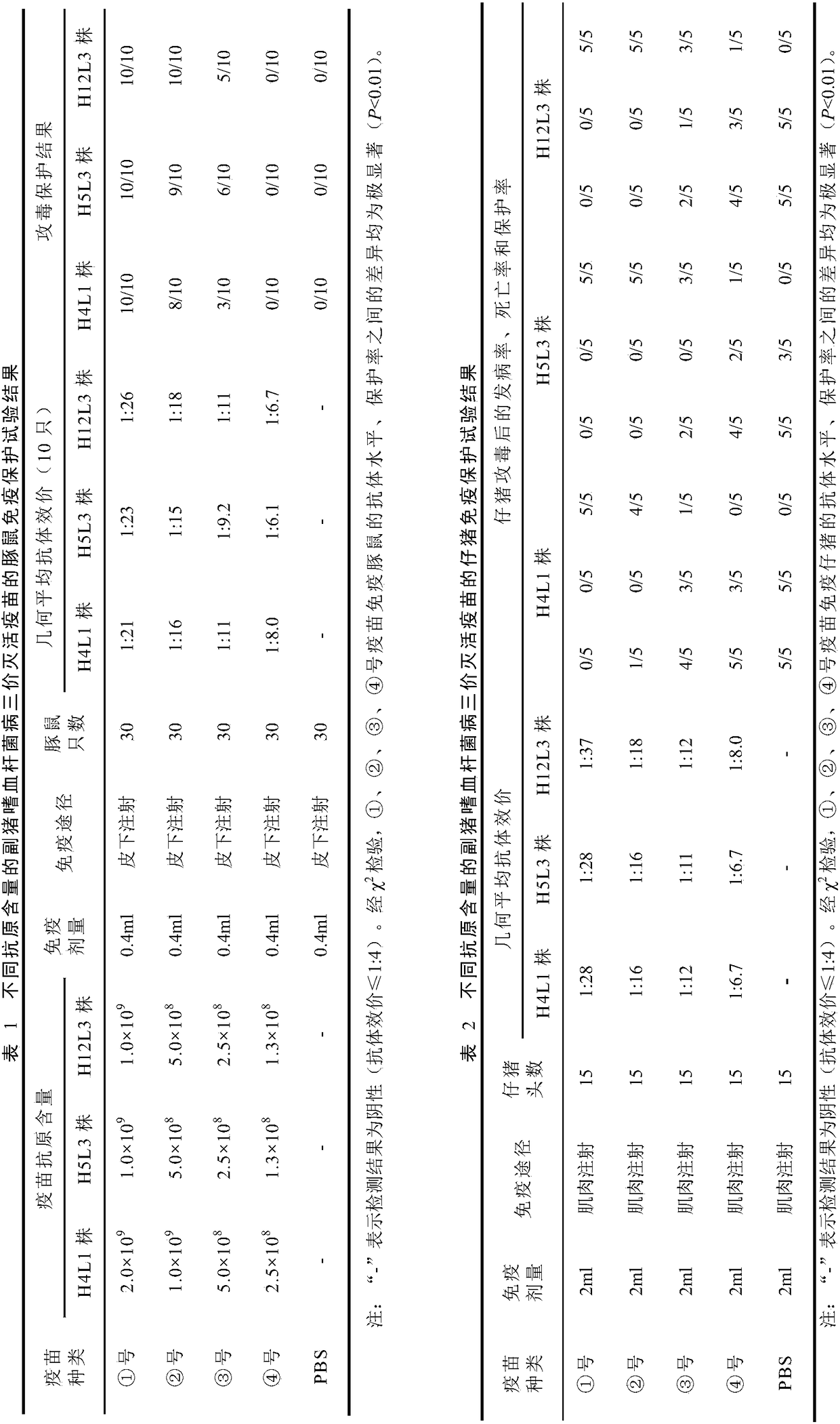
The invention discloses a haemophilus parasuis trivalent inactivated vaccine as well as a production method and application thereof. The haemophilus parasuis trivalent inactivated vaccine contains a haemophilus parasuis type-4 H4L1 strain, a type-5 H5L3 strain and a type-12 H12L3 strain being inactivated by a formaldehyde solution, as well as a water-based immunologic adjuvant, wherein the haemophilus parasuis type-4 H4L1 strain, the type-5 H5L3 strain and the type-12 H12L3 strain are all collected in the China Center for Type Culture Collection on 11 January, 2018 with the collection numbersof CCTCC M 2018019, CCTCC M 2018020 and CCTCC M 2018021 respectively. The trivalent inactivated vaccine disclosed by the invention is used for preventing the haemophilus parasuis disease caused by thetype-4, type-5 and type-12 haemophilus parasuis, and has the advantages of high security, high immune efficacy, and long immunity period and the like.
Owner:HENAN UNIV OF SCI & TECH +1
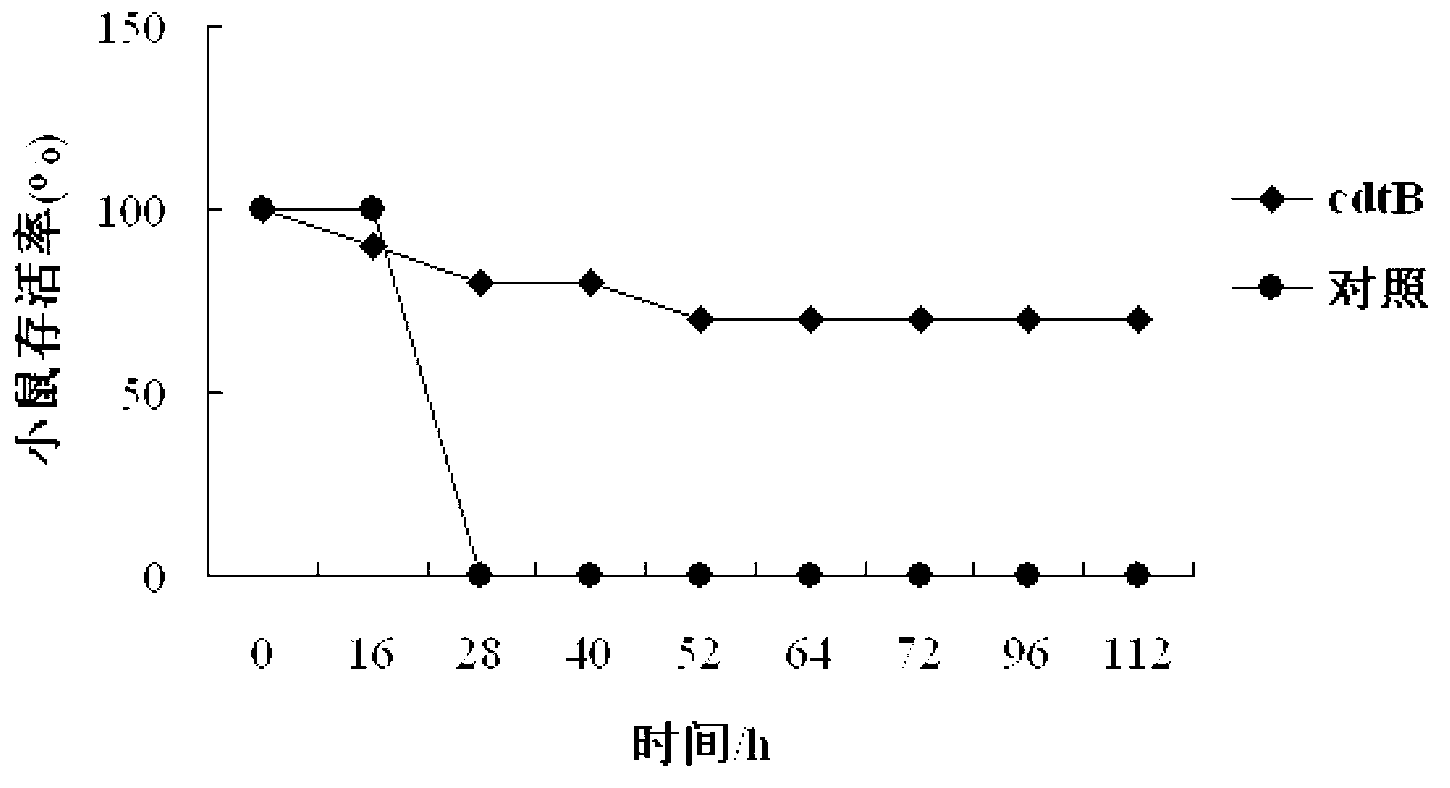
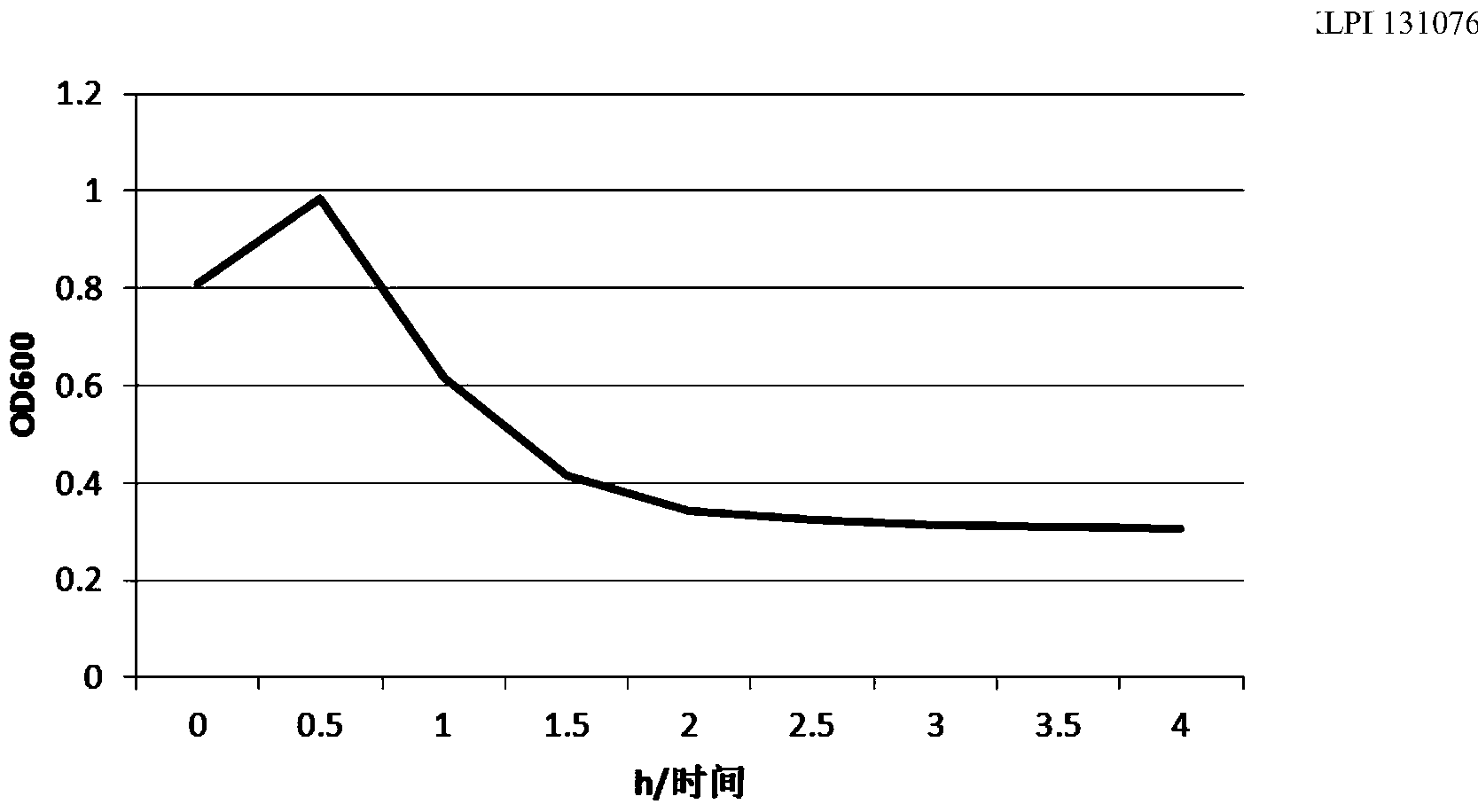
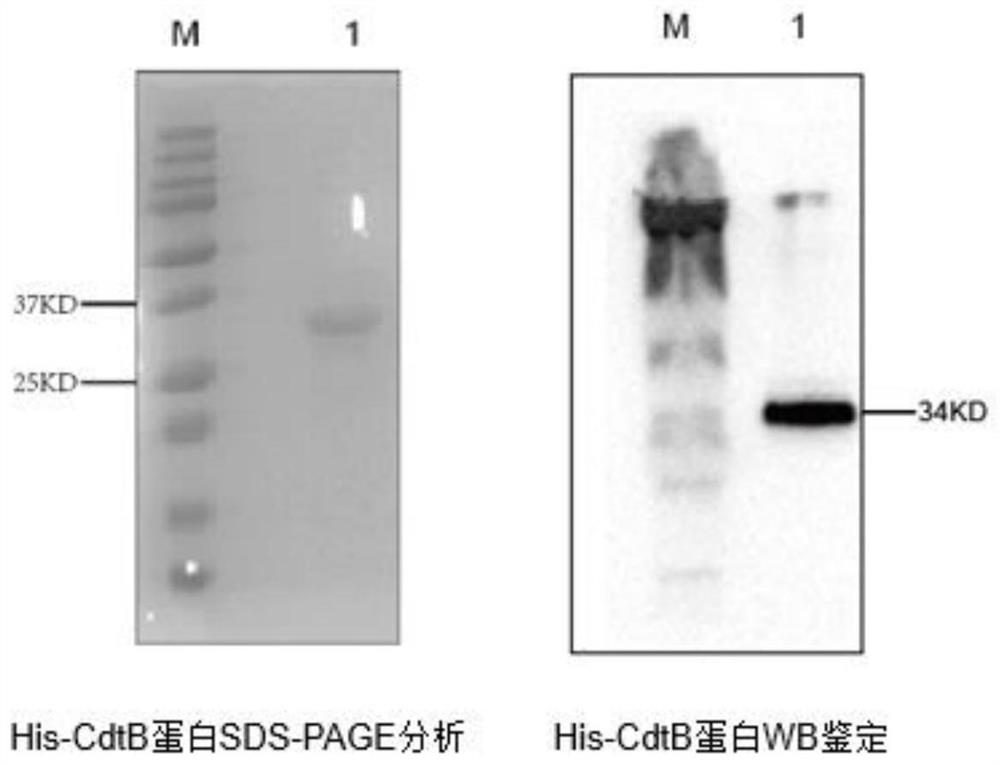
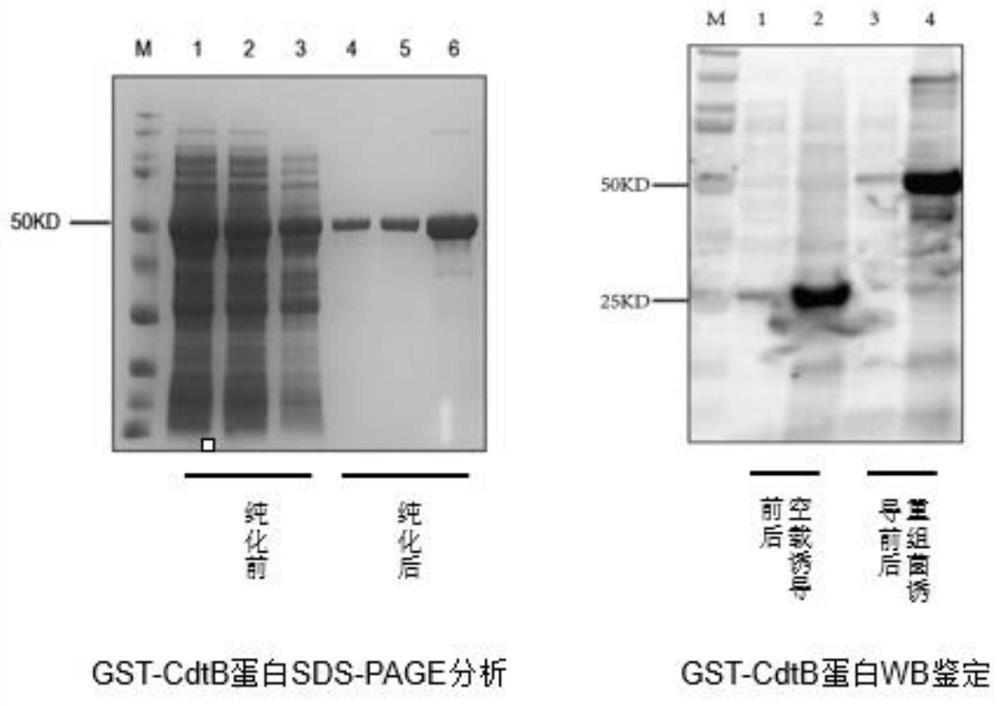
Haemophilus parasuis (Hps) immunoprotecive antigen CdtB
The invention relates to identification of a Haemophilus parasuis (Hps) immunoprotecive antigen, separation and cloning of a protein gene and application of a protein coded by the Hps immunoprotecive antigen in a vaccine. According to the invention, a new protein CdtB having immunogenicity is separated from Hps (the culture collection number is CVCC3361), and the nucleotide sequence is shown as SEQ ID NO:2 in a sequence table and is formed by coding 277 amino acids. The CdtB is a new protein having immunogenicity and can provide effective immunoprotection for Hps infection in mice. The invention also comprises preparation of an Escherichia coli recombinant bacterium BL21 / Hps-CdtB expressing the immunogenicity protein gene CdtB. The recombinant Hps CdtB protein expressed by the invention has favorable safety and protection efficacy, and the immunoprotection effect is up to 70%.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Haemophilus parasuis polypeptides and methods of use
The present invention provides isolated polypeptides having oligopeptide permease activity and an amino acid sequence that has at least 80% identity with a Haemophilus parasuis OppA polypeptide. Also provided by the present invention are isolated polynucleotides that encode the polypeptides described herein, and antibody that specifically binds a polypeptide described herein. The present invention further provides genetically modified microbes, such as attenuated Haemophilus parasuis strains and other microbes that express polypeptides described herein. Also included are methods for using the polypeptides, polynucleotides, antibody, and genetically modified microbes.
Owner:RGT UNIV OF MINNESOTA
Treble PCR method for simultaneously detecting mycoplasma hyopneumoniae, porcine pasteurella multocida and haemophilus parasuis
InactiveCN104263845AQuick checkShorten detection timeMicrobiological testing/measurementGenotypeP. multocida
The invention discloses a treble PCR method for simultaneously detecting mycoplasma hyopneumoniae, porcine pasteurella multocida and haemophilus parasuis. A treble PCR detection method for directly detecting 3 pathogens once from a sample is established through the following steps: firstly, screening out conservative genetic fragments with the gene type characteristics of the pathogens, using the conservative genetic fragments as 3 gene target points for PCR detection, respectively synthetizing and amplifying primers of the target point genes, and then putting 3 pairs of the primers of the 3 genetic fragments in a PCR reaction system. Through the adoption of the treble PCR method disclosed by the invention, on one hand, the pathogens can be accurately detected, and a mixed infection condition can be analyzed, so that the epidemic and development trend of an epidemic situation can be controlled, and the treble PCR method has double effects; on the other hand, compared with the conventional PCR method, the detecting time is shortened by 24 hours, so that the purpose of quickly detecting actual samples is achieved, and the cost is reduced.
Owner:SHANGHAI JIAO TONG UNIV
Haemophilus parasuis outer membrane protein P5 (OMP5) resistant monoclonal antibody, hybridoma cell strain and application
InactiveCN102876635ANo cross reactionStrong specificityImmunoglobulins against bacteriaMicroorganism based processesEscherichia coliBordetella
The invention discloses a haemophilus parasuis outer membrane protein P5 (OMP5) resistant monoclonal antibody, a hybridoma cell strain and an application. The hybridoma cell strain is preserved in the China center for type culture collection (CCTCC), and the preservation serial number is CCTCCC2012135. The monoclonal antibody prepared by the hybridoma cell strain is good in specificity, high in valence, high in generality, free from cross reaction with swine Escherichia coli, swine pasteurella, swine pleuropneumonia actinobacillus, streptococcus suis and swine bordetella bacilli, capable of detecting haemophilus parasuis with different serotypes and widely applicable to etiology diagnosis, serology detection and immunology detection and prevention of haemophilus parasuis diseases, and the enzyme-linked immuno sorbent assay (ELISA) antibody valence can reach 1:204800 after purification.
Owner:广东省农业科学院兽医研究所
Haemophilus parasuis (Hps) immunoprotecive antigen OppA
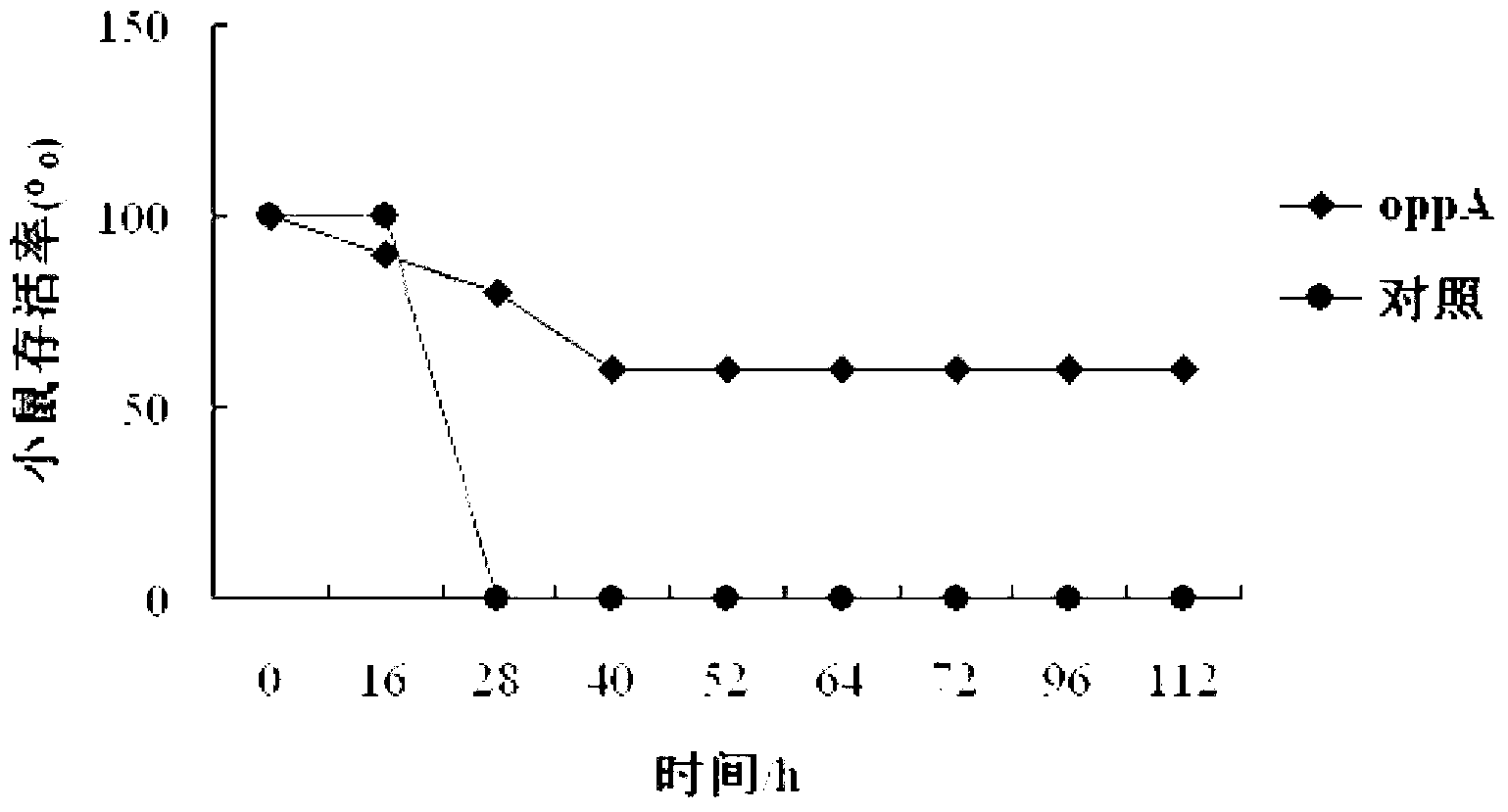
The invention relates to identification of a Haemophilus parasuis (Hps) immunoprotecive antigen, separation and cloning of a protein gene and application of a protein coded by the Hps immunoprotecive antigen in a vaccine. According to the invention, a new protein OppA having immunogenicity is separated from Hps (the culture collection number is CVCC3361), and the nucleotide sequence is shown as SEQ ID NO:2 in a sequence table and is formed by coding 545 amino acids. The OppA is a new protein having immunogenicity and can provide effective immunoprotection for Hps infection in mice. The invention also comprises preparation of an Escherichia coli recombinant bacterium BL21 / Hps-oppA expressing the immunogenicity protein gene oppA. The recombinant Hps OppA protein expressed by the invention has favorable safety and protection efficacy, and the immunoprotection effect is up to 60%.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Construction method of antibacterial cefquinome PK/PD model for livestock and application
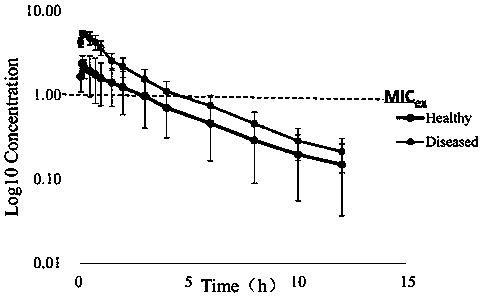
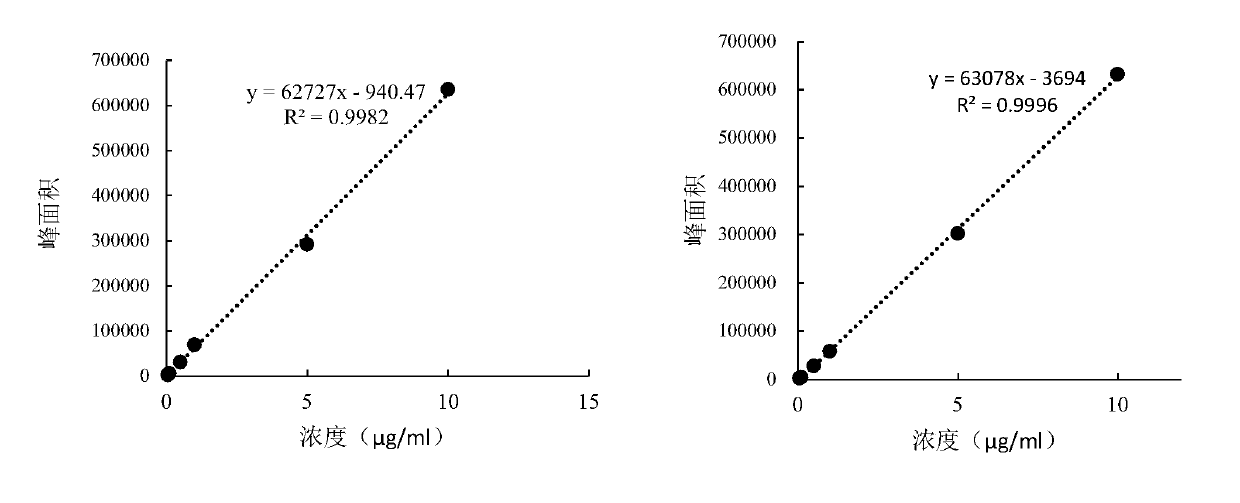
PendingCN110607344ADelay drug resistanceMolecular designMicrobiological testing/measurementAntibacterial actionDrug administration
The invention discloses a construction method of an antibacterial cefquinome PK / PD model for livestock and an application. The specific contents of the construction method comprise detecting the drugsusceptibility of antibacterial cefquinome to haemophilus parasuis so as to obtain an MIC distribution range; determining the concentrations of free drugs in plasma samples of healthy model animals and disease model animals at different time points after drug administration to obtain drug-time curves by a liquid chromatography detection method; performing fitting on pharmacokinetics parameters ofdrugs by pharmacokinetics software to obtain PK parameters; researching the antibiotic action of the antibacterial on pathogenic bacteria under the condition of in vitro and half in vivo, and performing fitting on the time limitation relationship of the antibacterial to the pathogenic bacteria under the condition of in vitro and half in vivo to obtain PD parameters; establishing a half in vivo PK-PD model according to a Sigmoid Emax equation; and obtaining drug administration schemes under different drug administration objectives through a dosage calculating formula and Mlxplore software. According to the construction method disclosed by the invention, an optimization method is provided for a drug resistance resistant medication scheme of the cefquinome clinically, and production and propagation of bacterial drug resistance are relieved.
Owner:HUAZHONG AGRI UNIV
Composition and method for identifying pasteurella multocida and/or haemophilus parasuis
ActiveCN106434935AGood repeatabilityStrong specificityMicrobiological testing/measurementMicroorganism based processesHaemophilusAgricultural science
The invention discloses a composition for identifying pasteurella multocida and haemophilus parasuis at the same time, a kit and an identifying method. The composition at least comprises the following two primer pairs, one primer pair is selected from at least one primer pair of a primer pair set composed of a PCR primer pairs composed of two single stranded DNAs shown in the sequence 1 and the sequence 2 in a sequence table and a PCR primer pair composed of two single stranded DNAs shown in the sequence 5 and the sequence 6 in the sequence table, and the other primer pair is selected from at least one primer pair of a primer pair set of a PCR primer pair composed of two single stranded DNAs shown in the sequence 3 and the sequence 4 in the sequence table, a PCR primer pair composed of two single stranded DNAs shown in the sequence 7 and the sequence 8 in the sequence table and a PCR primer pair composed of two single stranded DNAs shown in the sequence 9 and the sequence 10 in the sequence table. Pathogene can be accurately identified, the mixed infection condition can be analyzed, the identifying speed is high, cost is low, and the application range is wide.
Owner:CHINA ANIMAL DISEASE CONTROL CENT
Veterinary compound hydrochloric acid injection and preparation method thereof
InactiveCN102151263AGood effectEasy to prepareAntibacterial agentsOrganic active ingredientsProtozoaDisease
The invention relates to veterinary compound hydrochloric acid injection and a preparation method thereof. The veterinary compound hydrochloric acid injection consists of oxytetracycline dihydrate injection, imidocarb and diclofenac sodium; the veterinary compound hydrochloric acid injection per 100 ml comprises the required raw materials of: 10-25g of oxytetracycline dihydrate injection, 1.5-5g of diclofenac sodium, 0.1-0.2g of imidocarb, 0.2-0.6g of sodium formaldehyde sulphoxylate, 5-15g of magnesium chloride, 5-13 ml of ethanolamine and 60-81 ml of organic solvents, wherein the allowance is water for injection. The veterinary compound hydrochloric acid injection has remarkable effect when being used for treating acute respiratory infection caused by eperythrozoon suis, babesiosis and other blood protozoa diseases, porcine respiratory disease complex (PRDC) and swine influenza virus (SIV), airway inflammation induced by porcine reproductive and respiratory syndrome (PRRS), haemophilus parasuis, pasteurella, pleuropneumonia and mycoplasma diseases; and the preparation method is simple and easy to operate, and is suitable for batch production.
Owner:XUCHANG TIANYUAN BIOLOGICAL TECH CO LTD
Haemophilus parasuis OppA recombinant protein antigen-containing forward haemophilus parasuis disease indirect hamagglutination diagnosis reagent and preparation method thereof
ActiveCN103193872AEasy to detectPracticalBacteriaMicroorganism based processesDiseaseHistone antigen
The invention discloses a haemophilus parasuis OppA recombinant protein antigen-containing forward haemophilus parasuis disease indirect hamagglutination diagnosis reagent and a preparation method thereof, wherein the reagent is fast and convenient to detect, and strong in practicability. The beneficial effects lie in that (1) the detection processes are simple and convenient, fast and accurate, and the practicability is strong, and stronger popularization and application values can be achieved; (2) the sensitivity is high, the specificity is strong and the stability is good; (3) the haemophilus parasuis 15 serotype antibody can be detected with the diagnosis reagent; and (4) the diagnosis reagent and the preparation method thereof are safe and convenient, have no need of complicated detection instruments and equipment, and the detection results are intuitive.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Cross primer amplification primer group for detecting haemophilus parasuis, kit and application
ActiveCN106834432ALow costReduce use costMicrobiological testing/measurementMicroorganism based processesNucleic acid detectionHaemophilus
The invention discloses a cross primer amplification primer group for detecting haemophilus parasuis, a kit and application. The primer group comprises a primer group with nucleotide sequences as shown in SEQ ID NO.1 to 6; the kit comprises the primer group and a nucleic acid test strip; an application method of the kit comprises the following steps of firstly preparing a cross primer amplification reaction system; directly reading after using the nucleic acid test strip for detecting a product obtained through thermostatic reaction, wherein a positive result is characterized by appearing two strips, one is located in a detection area, and the other one is located in a quality control area. The kit is simple to operate, low in cost, easy to observe the reaction result, good in specificity, applicable for on-site test of export quarantine, food sanitation and an animal husbandry farm, and easy to popularize and apply in large scale.
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI
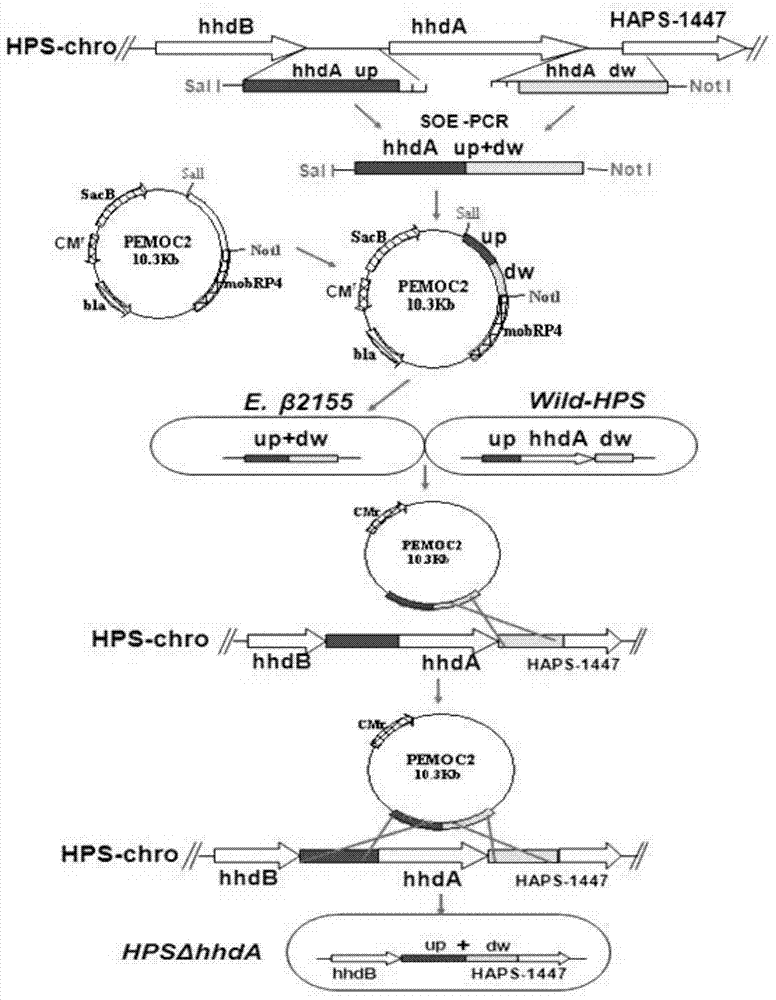
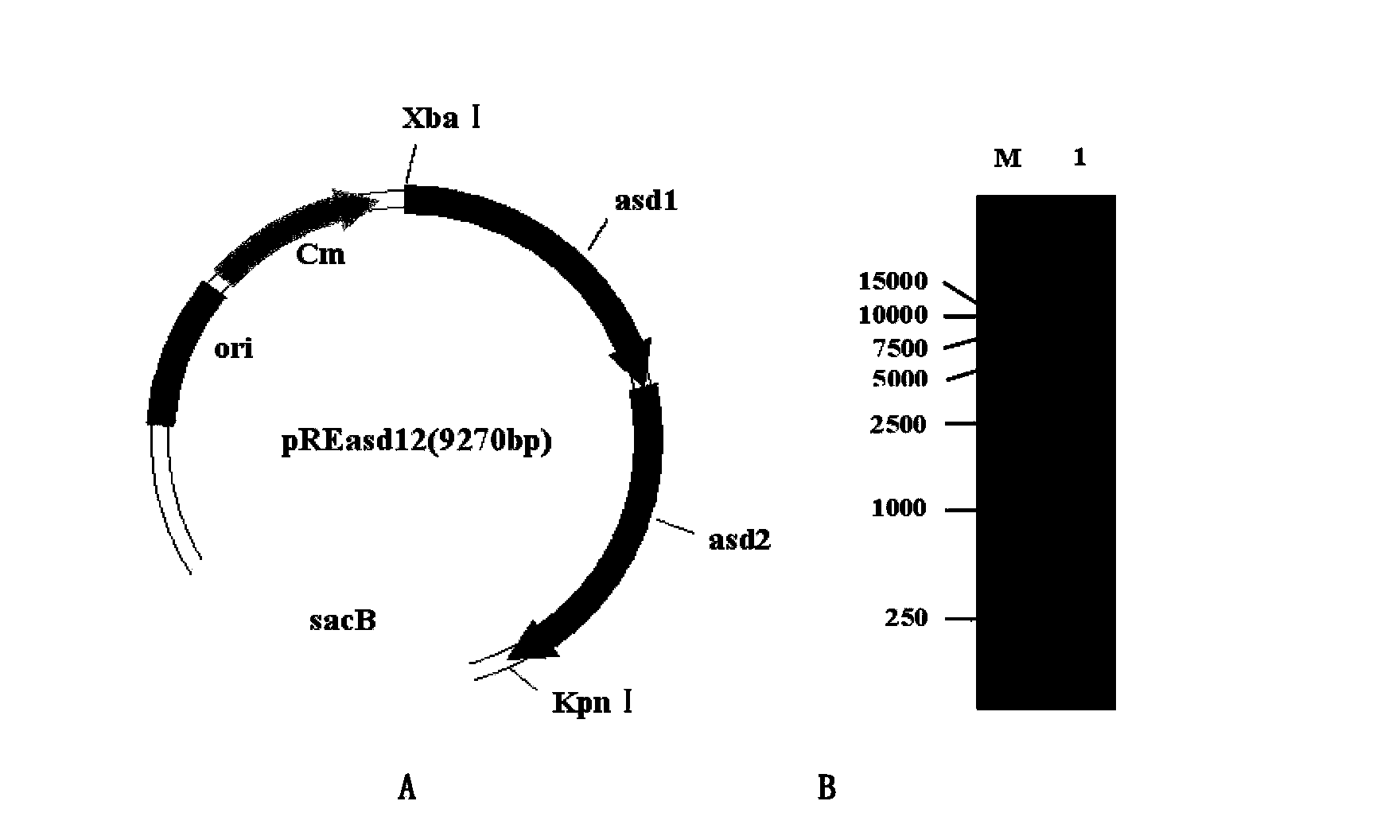
Haemophilus parasuis engineering strain with hhdA gene deletion and without resistance maker and construction method thereof
The invention discloses a haemophilus parasuis (HPS) strain with hhdA gene deletion and without a resistance maker and a construction method thereof. The construction method comprises the following steps: connecting upstream and downstream sequences of an hhdA gene of an HPS wild type strain by an overlapping PCR (Polymerase Chain Reaction) technology; constructing to a suicide plasmid vector (PEMOC2 delta hhdA) and transferring into an E.beta 2155 strain; transferring the PEMOC2 delta hhdA into the HPS wild type strain by a conjugal transfer method to screen out a single-exchanged positive combined son strain; carrying out limiting dilution continuous cultivation; and screening to obtain HPS delta hhdA of the HPS strain with hhdA gene deletion and without the resistance maker. The HPS delta hhdA strain constructed by the method has the growth characteristics and protective immunogenicity similar to those of a parent strain; an animal subjected to HPS delta hhdA strain immunizing does not produce an antibody for resisting hhdA protein, so that the HPS delta hhdA strain can be used as a vaccine for distinguishing the wild type strain in the processes of preventing, controlling and detecting haemophilus parasuis.
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI
Production method of haemophilus parasuis/mycoplasma hyopneumoniae bivalent inactivated vaccine
ActiveCN109010814AEasy to controlReduce controlAntibacterial agentsBacterial antigen ingredientsSerum igeHaemophilus
The invention belongs to the technical field of vaccine production, and particularly discloses a production method of a haemophilus parasuis / mycoplasma hyopneumoniae bivalent inactivated vaccine. Themethod includes: performing fermenting culture of mycoplasma hyopneumoniae in a bioreactor; performing fermenting culture of serum IV type haemophilus parasuis and serum V type haemophilus parasuis ina fermentation tank; inactivating, concentrating and purifying the microbial liquids after the fermenting cultivation, mixing the microbial liquids according to certain ratio, and adding an immuno-enhancer and a vaccine adjuvant to obtain the haemophilus parasuis / mycoplasma hyopneumoniae bivalent inactivated vaccine. The vaccine composition has excellent specificity and good immunity, can achievethe object of prevention from the two diseases by one injection, is more economical and practicability, can avoid repeated inoculation and reduces vaccine cost and manpower cost. The bivalent inactivated vaccine is very suitable for prevention and treatment on breeding farms with mixed infection of the diseases.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Cross-reactive determinants and methods for their identification
ActiveUS20110014225A1Antibacterial agentsBacterial antigen ingredientsCross-reactivityInfectious agent
Compositions and methods for determining immunologically cross-reactive molecules comprising a cross-reactive antigenic determinant are provided, in particular for determining proteins comprising cross-reactive antigenic determinants, in particular for determining proteins that are cross-reactive based on serological screens using sequential immunological challenges to an animal, including determining cross-reactive H. parasuis proteins. Also provided are compositions, vaccines, and kits using the molecules for diagnostics and methods for preventing or treating a disease, disorder, condition, or symptoms thereof associated with infectious agents, in particular infectious microorganisms, in particular for preventing or treating a disease, disorder, condition, or symptom thereof associated with H. parasuis infection.
Owner:ELANCO TIERGESUNDHEIT AG
Haemophilus parasuis, streptococcus suis and actinobacillus pleuropneumoniae triple inactivated vaccine and application thereof
PendingCN110075289AStrong pathogenicityImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsDiseaseAntigen
The invention relates to the technical field of animal biological products and specifically relates to a haemophilus parasuis, streptococcus suis and actinobacillus pleuropneumoniae triple inactivatedvaccine. The inactivated vaccine includes an inactivated haemophilus parasuis HNHPS1 serotype antigen, an inactivated haemophilus parasuis strain HN1570 serotype antigen, an inactivated streptococcussuis strain HNSS1 serotype antigen and an inactivated actinobacillus pleuropneumoniae strain HNAPP1 antigen; the four strains all have excellent immunogenicity; when the four strain inactivated antigens are used for preparing the vaccine, the infection of clinic haemophilus parasuis, streptococcus suis and actinobacillus pleuropneumoniae can be effectively prevented; the vaccine is characterizedby simple preparation process, high safety, excellent immune effect and long immunity period and meanwhile is capable of achieving the purposes of preventing various diseases with one injection, reducing cost and reducing stress.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE HENAN ACAD OF AGRI SCI
Method for preparing bacterial ghost vaccine of haemophilus parasuis as well as product and application thereof
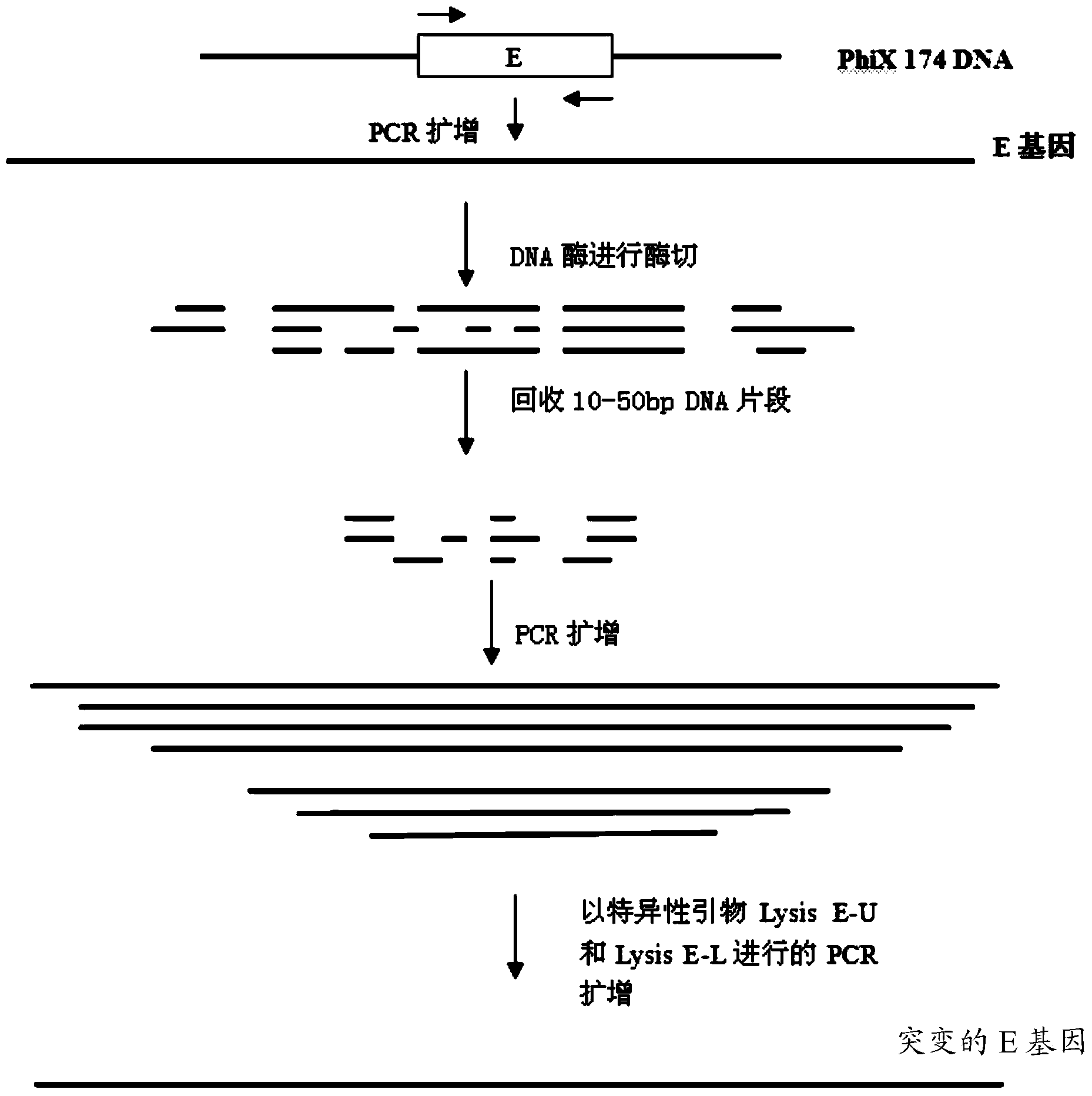
InactiveCN103446581ARepress transcriptionImprove cracking efficiencyAntibacterial agentsMicroorganism based processesEPROMHaemophilus
The invention discloses a method for preparing a bacterial ghost vaccine of haemophilus parasuis as well as a product and application thereof. The method comprises the following steps of connecting a mutational bacteriophage splitting gene E Eprom (as shown in SEQ ID No:1) with pBV220 to obtain an efficient splitting plasmid vector pBV-Eprom; converting the pBV-Eprom into haemophilus parasuis, propagating at 37 DEG C, and inducing the Eprom gene to express at 42 DEG C, and collecting the product which is unexpressed finally to obtain haemophilus parasuis bacterial ghost, wherein the Eprom is obtained by carrying out mutation on a promoter region of the bacteriophage splitting gene E, and the temperature for culturing bacteria is changed to 37 DEG C from the existing 28 DEG C by the Eprom; moreover, the splitting efficiency is high, the initial induced concentration and the large-scale production capacity are high, and the culture-splitting efficiency of a fermentation tank is as high as 99.99995%. The bacterial ghost vaccine of the haemophilus parasuis disclosed by the invention has good safety and immune protective efficacy, can be used for stimulating a body to generate a high-titration antibody, and also can be used for providing good cross immune protection for attack of a virulent strain of the haemophilus parasuis with different serotypes.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Application of haemophilus parasuis CdtB hybridoma cell and monoclonal antibody
ActiveCN111793130AHighly conservativeImprove versatilityAntibacterial agentsBiological material analysisInfectious DisorderSerotype
The invention discloses application of a haemophilus parasuis CdtB hybridoma cell and a monoclonal antibody, and belongs to the field of detection, diagnosis and treatment of animal infectious diseases. The preservation number of the haemophilus parasuis CdtB hybridoma cell is CCTCC NO:C201910, the subclass of the monoclonal antibody generated by the haemophilus parasuis CdtB hybridoma cell is IgG1, the light chain type is kappa, and the recognized epitope amino acid sequence is highly conservative in haemophilus parasuis. The monoclonal antibody disclosed by the invention is high in titer, can identify all serotype haemophilus parasuis, and also has neutralizing activity against CdtB. The monoclonal antibody and the epitope amino acid sequence recognized by the monoclonal antibody can bewidely applied to etiological diagnosis of haemophilus parasuis, serological diagnosis, immunological detection, disease prevention and treatment, wild virus infection and vaccine distinguishing and the like of haemophilus parasuis, and are used for preparing products related to the application.
Owner:HUAZHONG AGRI UNIV
Porcine circovirus type 2, mycoplasma hyopneumoniae and haemophilus parasuis triple inactivated vaccine and preparation method thereof
InactiveCN110812474AHigh activityGood immune effectAntibacterial agentsBacterial antigen ingredientsHemophilus parasuisHaemophilus species
The invention discloses a porcine circovirus type 2, mycoplasma hyopneumoniae and haemophilus parasuis triple inactivated vaccine and a preparation method thereof, which belong to the technical fieldof veterinary biological products. The vaccine consists of an antigen and a vaccine adjuvant, wherein the antigen consists of a porcine circovirus type 2 antigen, a mycoplasma hyopneumoniae antigen and a haemophilus parasuis antigen; the porcine circovirus type 2 antigen is cap protein obtained through expression of pichia pastoris, and the protein content is larger than or equal to 150 microgramsper milliliter, the mycoplasma hyopneumoniae antigen is inactivated mycoplasma hyopneumoniae bacterial liquid; wherein the haemophilus parasuis is an inactivated haemophilus parasuis liquid; a vaccine adjuvant is composed of a water-based high-molecular polymer adjuvant and a composite polysaccharide immunopotentiator. The triple inactivated vaccine for the porcine circovirus type 2, the mycoplasma hyopneumoniae and the haemophilus parasuis provided by the invention has obvious advantages in preventing the three swine diseases and improving the swine production performance.
Owner:山东滨州沃华生物工程有限公司
Streptococcus suis disease-haemophilus parasuis disease-porcine infectious pleuropneumonia triple subunit vaccine and preparation method thereof
ActiveCN113350495AImprove protectionStrong immune responseAntibacterial agentsBacterial antigen ingredientsAntigenDisease
The invention discloses a streptococcus suis disease-haemophilus parasuis disease-porcine infectious pleuropneumonia triple subunit vaccine and a preparation method thereof. The triple subunit vaccine contains streptococcus suis antigen proteins MRP, SLY and EF, haemophilus parasuis antigen proteins AfuA, OppA2, CdtB and OppA, and actinobacillus pleuropneumoniae antigen proteins ApxI, ApxII and OMP. Experiments prove that the vaccine can stimulate strong immune response of mice, and has a good cross protection effect on streptococcus suis, haemophilus parasuis and actinobacillus pleuropneumoniae challenge mice with different serotypes, which fully shows that the triple subunit vaccine prepared in the invention has a good protection effect. Therefore, the invention lays a solid foundation for developing an efficient, broad-spectrum and cheap streptococcus suis disease-haemophilus parasuis disease-porcine infectious pleuropneumonia vaccine, and provides an effective technical means for preventing and treating streptococcus suis disease, haemophilus parasuis disease and porcine infectious pleuropneumonia.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Attenuated vaccine of salmonella choleraesuis capable of expressing surface antigen of haemophilus parasuis
ActiveCN103421732AGood immune protectionEasy to operateAntibacterial agentsBacterial antigen ingredientsAntigenBacterial disease
The invention belong to the field of gene engineering vaccines for animal bacterial diseases, and particularly relates to construction, vaccine preparation and application of an attenuated vaccine strain of recombined salmonella choleraesuis capable of expressing a surface antigen gene HbpB of haemophilus parasuis without resistance markers. The recombined salmonella choleraesuis asd-C500 / pYA-HbpB capable of the expressing surface antigen gene HbpB of hemophilus parasuis without resistance markers is obtained (the preservation number is CCTCC No: M2013052); the asd gene on the genome of the salmonella choleraesuis is lost by the recombined strain, so that the asd gene and the recombinant plasmid pYA-HbpB of an outer membrane antigen of haemophilus parasuis can both be expressed in the strain. The invention further discloses a construction method of the recombination strain asd-C500 / pYA-HbpB and a corresponding manufacturing method of the attenuated vaccine of the recombination strain asd-C500 / pYA-HbpB, and application to the preparation of vaccines of salmonella choleraesuis and haemophilus parasuis.
Owner:HUAZHONG AGRI UNIV
Streptococcus suis and haemophilus parasuis bivalent inactivated vaccine and preparation method thereof
PendingCN114181846ALittle side effectsImprove securityAntibacterial agentsBacterial antigen ingredientsAntigenAdjuvant
The invention discloses a streptococcus suis and haemophilus parasuis combined inactivated vaccine and a preparation method thereof. The streptococcus suis and haemophilus parasuis combined inactivated vaccine comprises an antigen and an adjuvant, the antigen comprises an inactivated antigen concentrated solution of a haemophilus parasuis serum type 4 YC strain, a haemophilus parasuis serum type 5 SQ strain, a streptococcus suis type 2 NT strain and a streptococcus suis type 9 CZ strain. The vaccine provided by the invention can be used for simultaneously preventing haemophilus parasuis disease caused by haemophilus parasuis, streptococcus suis disease caused by streptococcus suis and mixed infection of the haemophilus parasuis disease and the streptococcus suis disease, is small in side effect and good in safety, can achieve the effect of preventing multiple diseases by one injection, and particularly can be used for preventing and treating infection of currently popular serotype germs; comprising infection of haemophilus parasuis type 4 and type 5 and infection of streptococcus suis type 2 and type 9.
Owner:JIANGSU NANNONG HI TECH
Quad inactivated vaccine of PCV2-type baculovirus, swine mycoplasma hyopneumoniae, porcine influenza virus and haemophilus parasuis
InactiveCN112386685AProlonged occurrenceExtension of timeSsRNA viruses negative-senseAntibacterial agentsAntigenAdjuvant
The invention belongs to the field of veterinary vaccines, particularly relates to a quad inactivated vaccine of PCV2-type baculovirus, swine mycoplasma hyopneumoniae, a porcine influenza virus and haemophilus parasuis and further discloses a preparation method for the quad inactivated vaccine and application of the quad inactivated vaccine. The quad inactivated vaccine disclosed by the inventioncontains inactivated protein Cap expressed by the PCV2-type baculovirus, inactivated swine mycoplasma hyopneumoniae, an inactivated porcine influenza virus H1N1subtype virus, inactivated haemophilus parasuis types 4, 5 and 13 and an adjuvant of the vaccine; four kinds of antigens are free of interference to one another, four kinds of protection can be achieved by one injection, and four kinds of epidemic diseases can be prevented through one-time immunization; and meanwhile, in view of toxic substance counteracting protection and serum antibody level, the immunization effect reaches or exceedsthat of each single commodity vaccine, the immunization duration is long, the efficacy is durable, and the quad inactivated vaccine has the advantages that the safety is good, the preparation methodis simple, the immunization is convenient, the immunization cost is reduced, and the like.
Owner:BEIJING KEMUFENG BIOLOGICAL PHARMA +3
Triple inactivated vaccine for haemophilus parasuis diseases, streptococcosis suis diseases and pasteurella multocida diseases of pigs and preparation method of triple inactivated vaccine
InactiveCN110812473ATargetedImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsStreptococcus suis serotypeAntigen
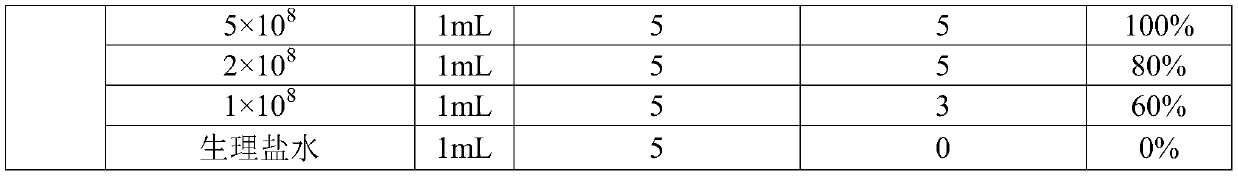
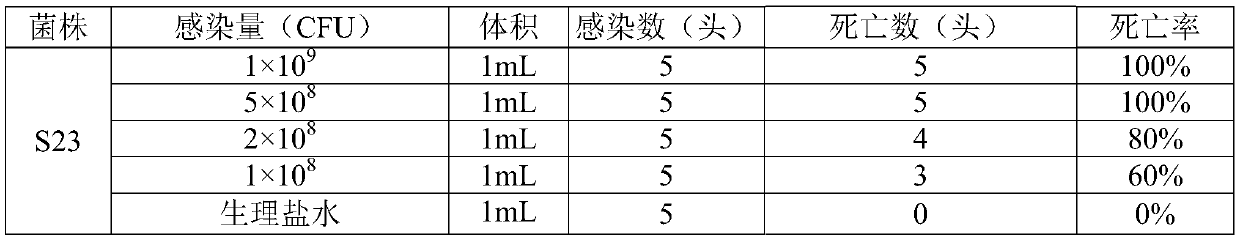
The invention belongs to the field of veterinary biological products and in particular relates to a triple inactivated vaccine for haemophilus parasuis diseases, streptococcosis suis diseases and pasteurella multocida diseases of pigs and a preparation method of the triple inactivated vaccine. The triple inactivated vaccine of haemophilus parasuis, streptococcosis suis and pasteurella multocida inpigs, which is provided by the invention, comprises an antigen concentrated solution and a nano aluminum adjuvant, wherein the antigen concentrated solution comprises a deactivated haemophilus parasuis serum type-4 H24 strain, a deactivated haemophilus parasuis serum type-5 H24 strain, a streptococcosis suis type-2 serum S23 strain, a pasteurella multocida type-A P13 strain and a pasteurella multocida type-B P11 strain. The triple inactivated vaccine is capable of effectively preventing haemophilus parasuis diseases, streptococcosis suis diseases and pasteurella multocida diseases of pigs, the purposes that multiple effects are achieved with one injection and stress is reduced are achieved, and the epidemic prevention cost is reduced.
Owner:广东君睿生物技术研究有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com