Haemophilus parasuis (Hps) immunoprotecive antigen OppA
A technology of Haemophilus suis and protective antigen, applied in the field of animal infectious disease subunit vaccine preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, the discovery of OppA protein immunogenicity
[0034] 1. Preparation of secreted protein samples
[0035] The strain of Haemophilus parasuis (Hps) (purchased from the National Center for Veterinary Microorganism Culture Collection, strain number: CVCC3361) was taken out from the refrigerator at -70°C, thawed at room temperature, and then streaked and inoculated in TSA (Becton, Dickinson and Company) solid medium (containing 10% horse serum, 0.01% NAD (nicotinamide adenine dinucleotide)), cultivated at 37°C for 36h, then picked a single colony and inoculated it in TSB (Becton, Dickinson and Company) liquid Culture medium (without horse serum, containing 0.02% NAD), cultured at 37°C for 24 hours, and then transferred to 500 mL TSB liquid medium (without horse serum, containing 0.02% NAD) with a volume of 1% bacterial solution, 37 Cultivate for about 16 hours at ℃ to make the OD of the bacterial solution reach 1.0. Add protease inhibitors to the bacterial c...
Embodiment 2
[0049] Embodiment 2, the expression of OppA protein
[0050] 1. Extraction of total Hps DNA
[0051] Centrifuge 1 mL of the overnight culture of Hps (CVCC3361) at 12,000 rpm for 1 minute, and discard the supernatant. Add 40 μL DB solution (TIANamp Bacteria DNA Kit, Tiangen Biological Co., Ltd.), 160 μL lysozyme and 8 μL RNaseA to the cell pellet. Shake vigorously to mix well. Incubate at 37°C for 30-60 minutes, and invert the centrifuge tube several times. Add 200 μL of DLT solution (TIANamp Bacteria DNA Kit, Tiangen Biological Co., Ltd.) and 25 μL of proteinase K (TIANamp Bacteria DNA Kit, Tiangen Biological Co., Ltd.), and immediately mix it gently by inversion. Place in a 65°C water bath for at least 30 minutes, and invert the centrifuge tube several times. Centrifuge at 12000rpm for 3-5 minutes, and pipette all the supernatant into a clean centrifuge tube. Add 200 μL of absolute ethanol, mix well, suck it into the adsorption column, centrifuge at 12000 rpm for 30 seco...
Embodiment 3
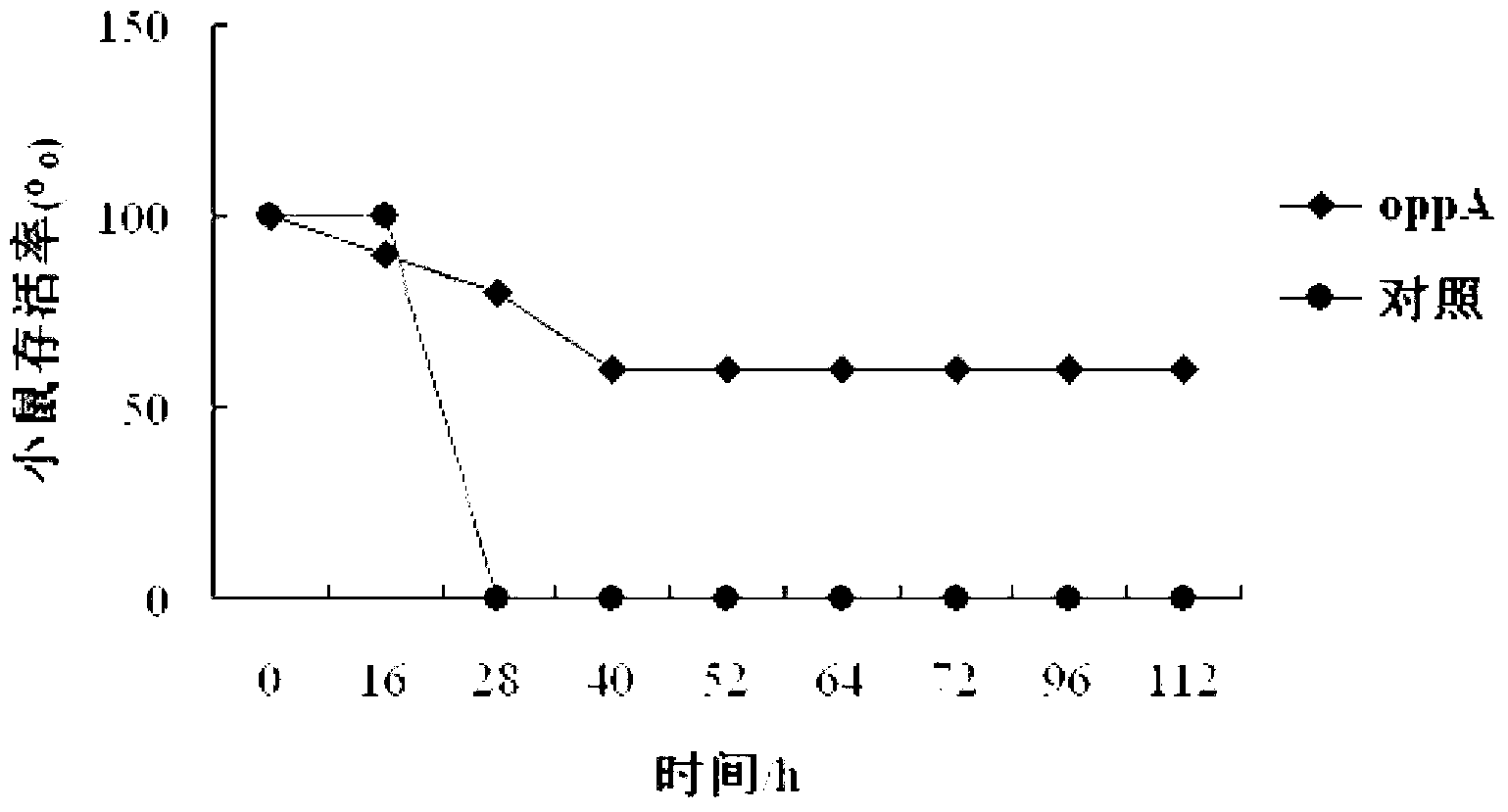
[0077] Example 3, Identification of OppA Protein Immunoprotection
[0078] 1. Effect of Hps on LD of Kunming mice 50 Determination of
[0079] Inoculate Hps (purchased from the National Center for Veterinary Microorganism Culture Collection, strain number: CVCC3361) in TSB liquid medium (containing 10% horse serum, 0.01% NAD) at 37°C at 200 rpm / min for 14 to 16 hours, and then the next day Spread TSA solid medium (containing 10% horse serum, 0.01% NAD) and incubate at 37°C for 24-36h. Wash the bacterial lawn with PBS and dilute to 5×10 8 CFU / mL (OD 600 about 1.0), then 2-fold concentrated step by step, concentrated to the required dose (as shown in Table 1) and then injected into mice. Female Kunming mice aged 8-10 weeks (purchased from the Experimental Animal Center of Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences) were divided into 5 groups, 10 mice in each group. Each mouse was intraperitoneally injected with 100 μL of the challenge dru...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com