Chicken Marek's disease Meq gene deleted vaccine strain, construction method thereof, and application thereof
A gene deletion vaccine, chicken Marek's disease technology, applied in the field of biomedicine to achieve stable genetics and great application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Recombinant virus transfer plasmid construction:
[0066] 1. Test materials: MDV MS strain (see literature: Construction of Marek's virus miRNA deletion strain and its growth in vitro, Yang Wenchuang, Liu Changjun, etc., Chinese Veterinary Science. 2011, 41 (03)), MDVMd5 (see literature: Marek virus supervirulent strains (Md5, RB1B) virulence test, Gan Junji Liu Xiufan Wu Changxin, Proceedings of the Eleventh Academic Symposium of the Poultry Disease Branch of the Chinese Society of Animal Husbandry and Veterinary Medicine. 2002), chicken Marek disease virus 814 strains (see literature : Cloning and sequence analysis of gE, gI, gp82 genes of chicken Marek's disease virus 814 strains. Zhang Yanping, Liu Changjun, etc. Advances in Animal Science, 2007 (3)) Preserved and provided by the Laboratory of Avian Infectious Diseases, Harbin Veterinary Research Institute; no specific pathogens (SPF) chicken embryos and chickens were provided by the Animal Center of Harbi...
Embodiment 2
[0075] Example 2 The acquisition and identification of recombinant virus-rMSΔMeq
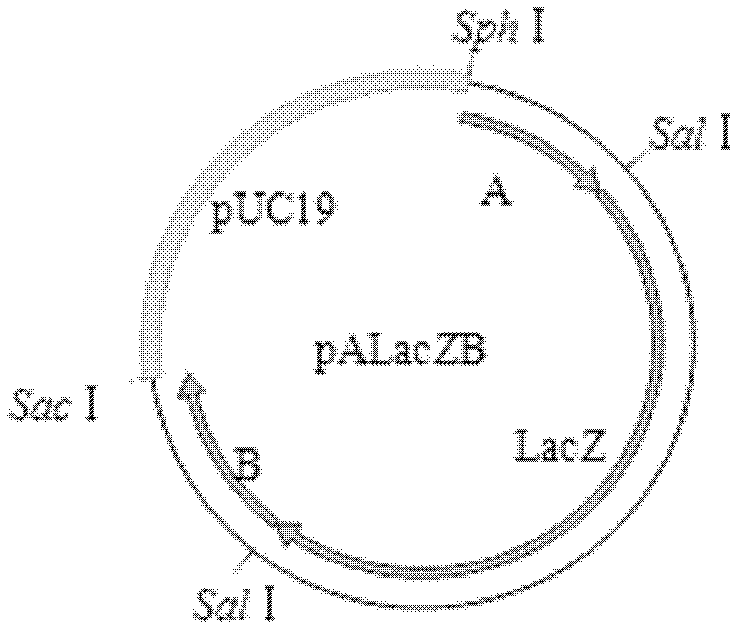
[0076] The first recombination: 1) Co-transfection: The total genomic DNA of MDV MS strain and the transfer vector plasmid pALacZB were co-transfected into CEF by calcium phosphate precipitation method. In the case of CEF, passage to a cell culture dish with a diameter of 60 mm after digestion, and the inoculum amount of CEF is 9×10 per dish. 6 indivual. First, add 388 μL of nuclease-free deionized water into a clean and sterile 1.5 mL centrifuge tube; then add 10–18 μL of the prepared MS strain-infected CEF genomic total DNA (0.8 μg μL-1) and plasmid pALacZB in sequence (1μg·μL-1)2μL; add 30~36μL TE (pH7.4); slowly add 62μL 2mol·L-1CaCl 2 , mix slowly; use a 1mL pipette to slowly add 500μL of 2×HEPES from the bottom, use a micropipette with a 200μL range to blow about 20 bubbles from the bottom to mix well, and incubate in a 37°C incubator for 30min. In the meantime, pack it into fine pellet...
Embodiment 3
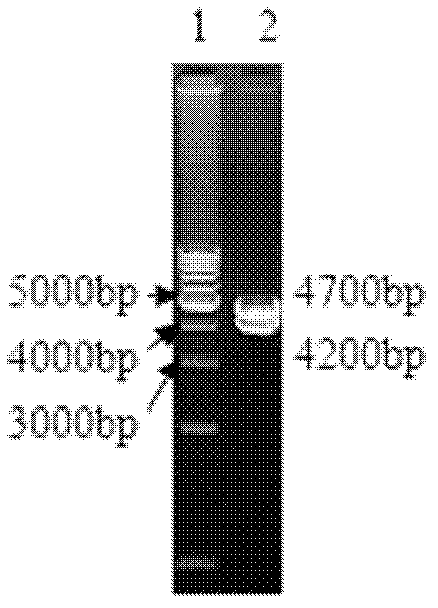
[0082] Embodiment 3 biological characteristics of recombinant virus
[0083] 1) Detection of growth characteristics of recombinant virus
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com