Streptococcus suis disease-haemophilus parasuis disease-porcine infectious pleuropneumonia triple subunit vaccine and preparation method thereof
A technology for Haemophilus suis disease and Haemophilus suis, which is applied in the field of triple subunit vaccine and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
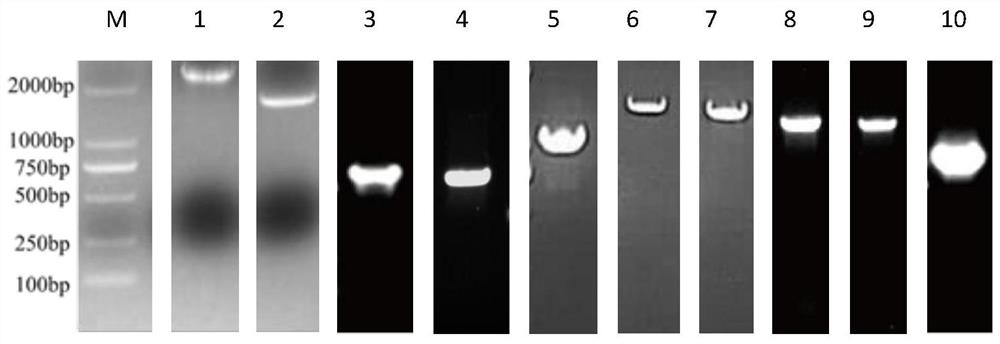
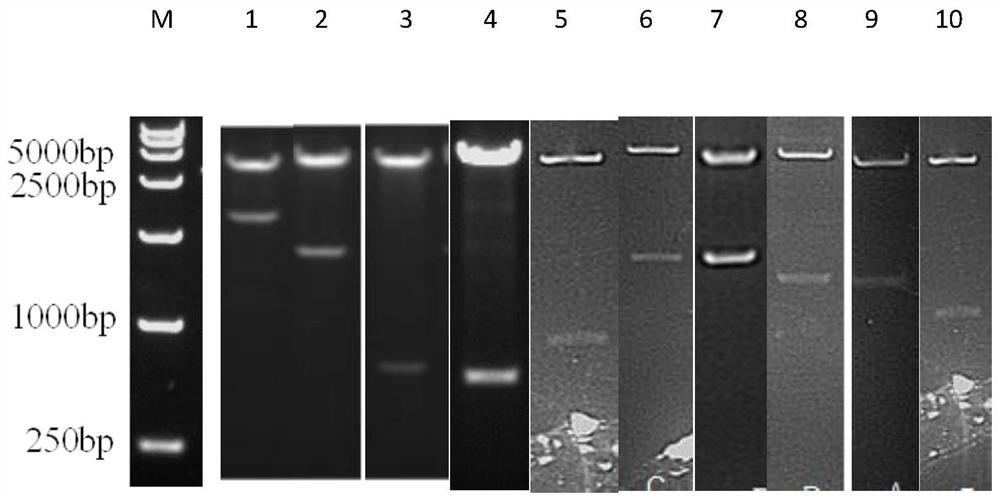
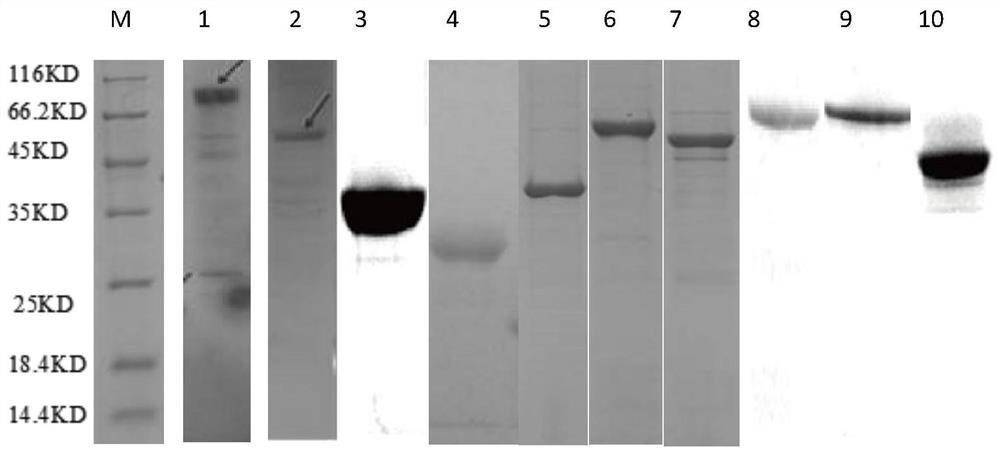
[0041] Expression and purification of embodiment 1 protein
[0042] 1 Extraction of bacterial genome
[0043] Streptococcus suis 05ZYH33 glycerol bacteria preserved in the laboratory were streaked in THA medium (containing 10% horse serum), then placed in a constant temperature incubator at 37°C overnight, and a single colony was picked and transferred to THB medium (containing 10% horse serum). % horse serum) in an incubator for static culture, and then expand the culture to 10mL THB medium (containing 10% horse serum), and use the bacterial genome extraction kit to extract the bacterial genome according to the instructions. The extracted genome was stored in a -20 refrigerator for future use.
[0044] Streak the Haemophilus parasuis HN10 glycerol bacteria preserved in the laboratory in TSA medium (containing 10 μg / ml NAD and 10% horse serum), then place it in a constant temperature incubator at 37°C overnight, and pick a single colony for transfer Put it into TSB medium (c...
Embodiment 2
[0066] Example 2 Preparation and Animal Test of Streptococcus Suis-Haemophilus Parasuis-Porcine Infectious Pleuropneumonia Triple Subunit Vaccine
[0067] 1 Preparation of triple subunit vaccine
[0068] Take the purified protein rMRP, rSLY, rEF, rCdtB, rAfuA, rOppA, rOppA2, rApxI, rApxII, rOMP mixed in Example 1, mix the mixed protein solution with ISA201VG in an equal mass ratio and emulsify, so that each dose of emulsified The content of each protein in the vaccine is 20 μg.
[0069] 2 Mouse Immunization Test
[0070] Twenty 4-week-old SPF C57 mice were randomly divided into 2 groups, 10 in each group, and 40 4-week-old SPF BALB / c mice were randomly divided into 2 groups, 20 in each group. Mice were immunized according to the immunization schedule in Table 5. The second immunization was carried out 14 days after the first immunization, and the dose and route of immunization were the same as those of the first immunization. 14 days after the second immunization, blood wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com