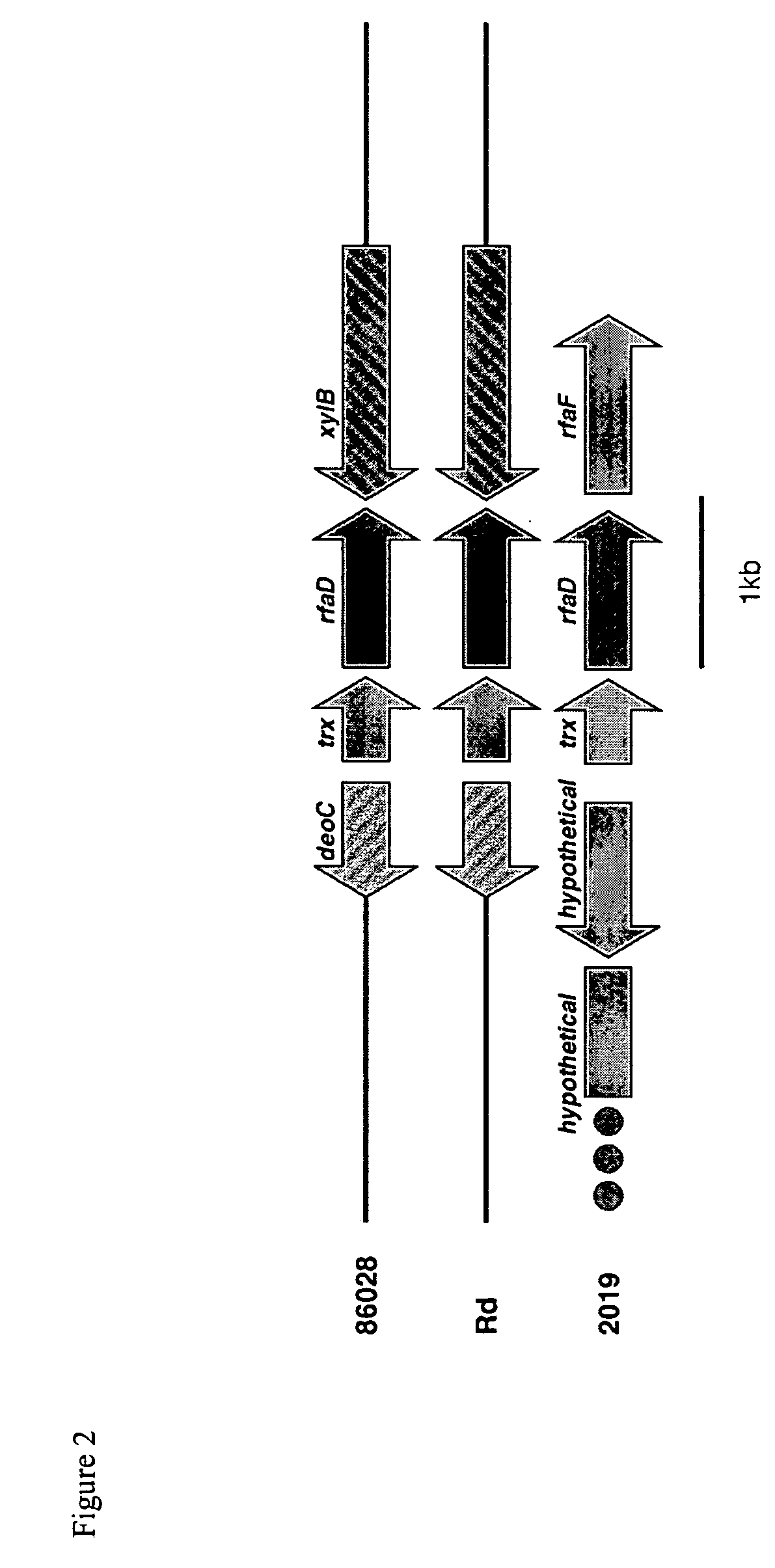
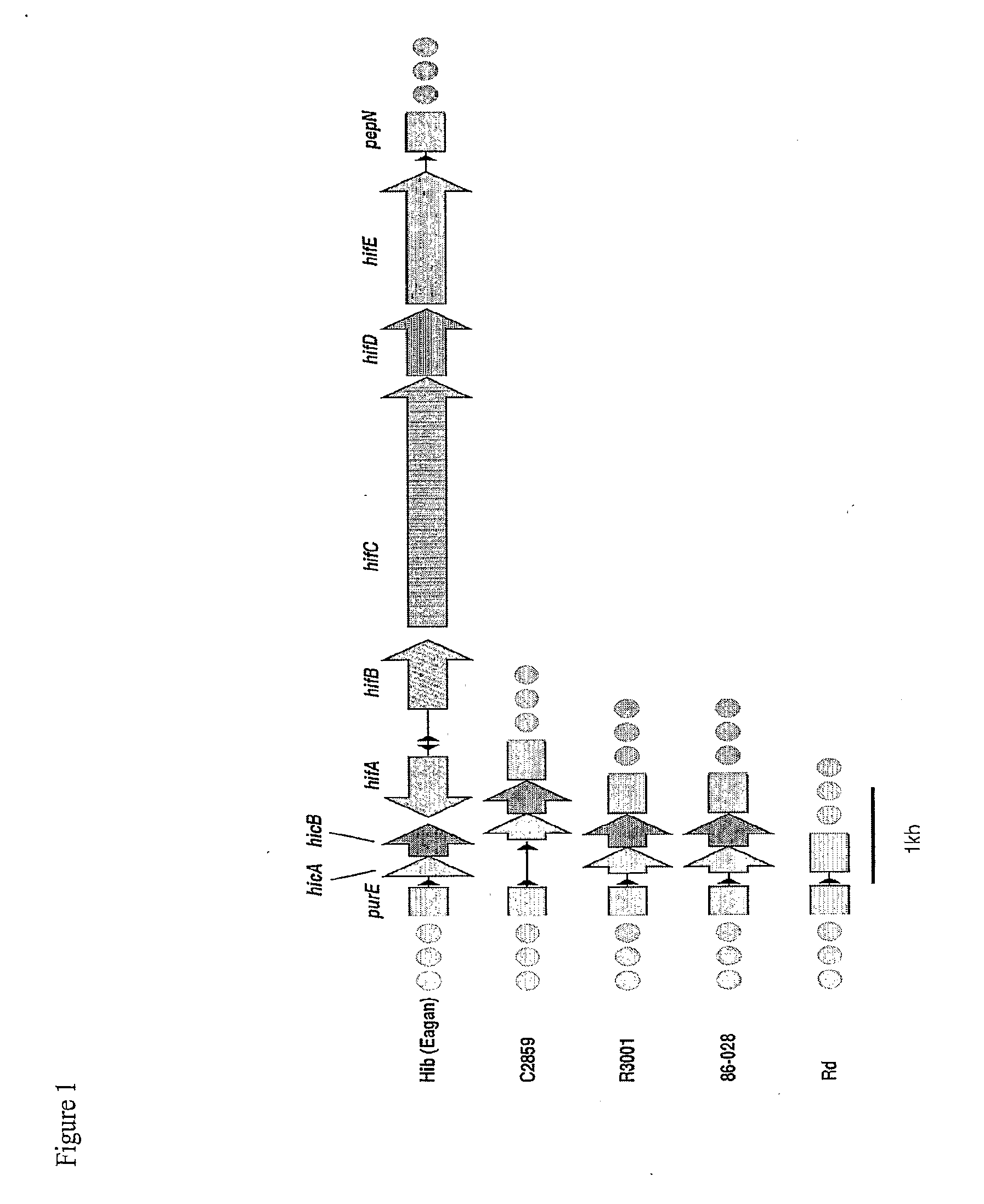
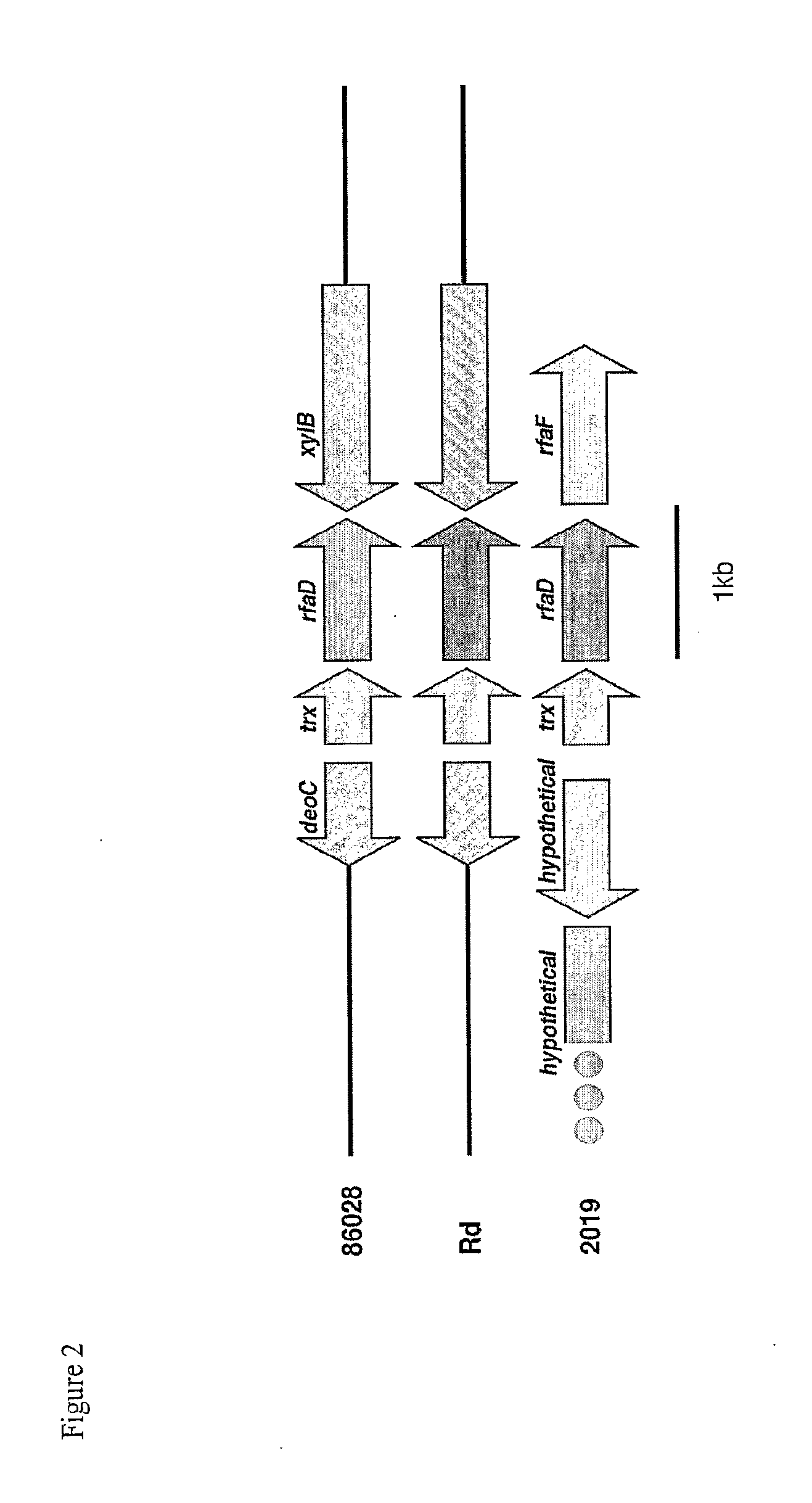
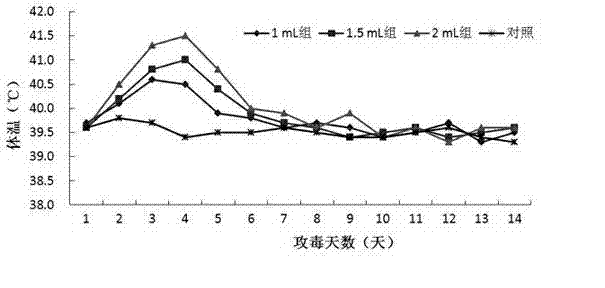
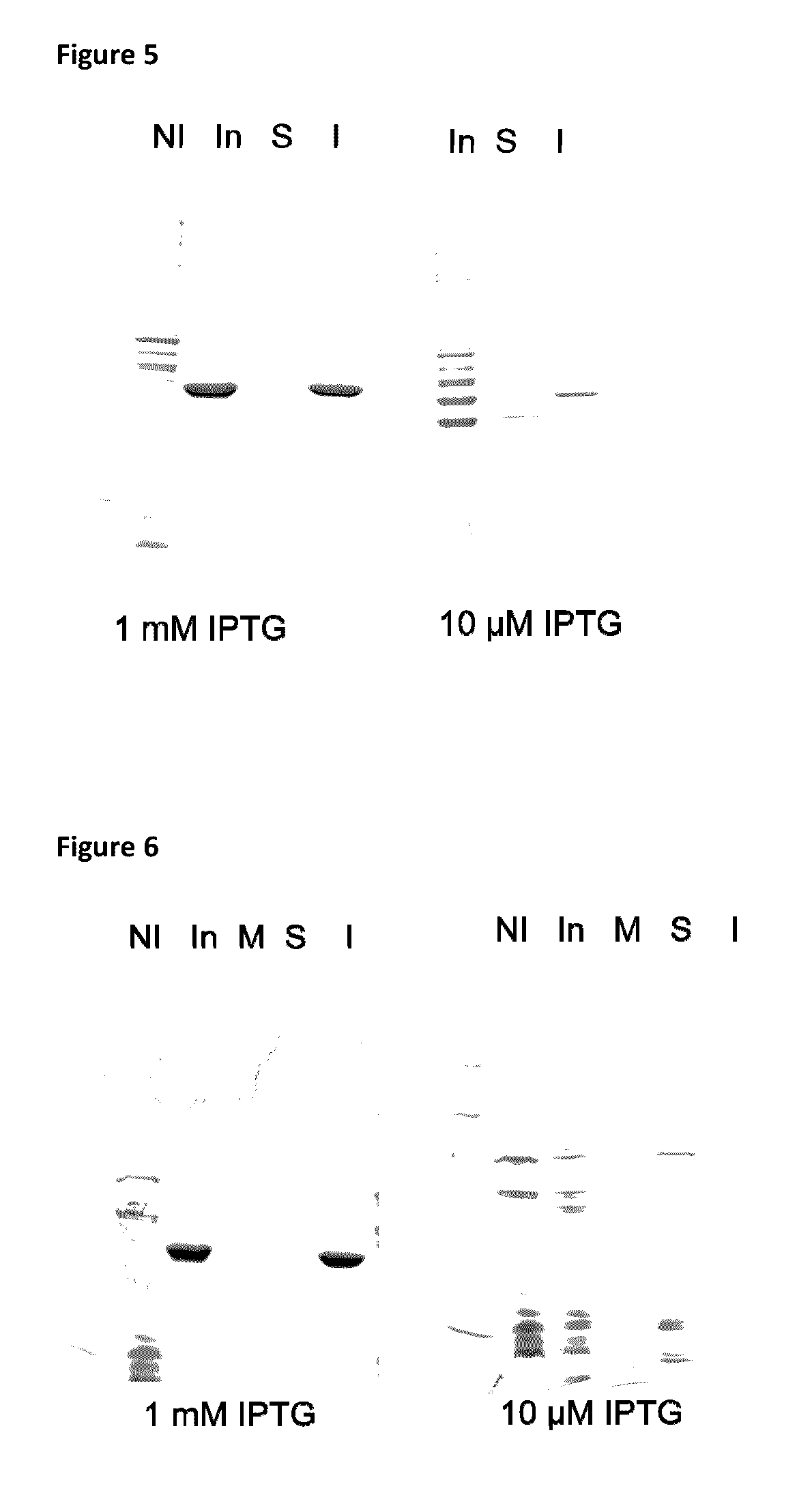
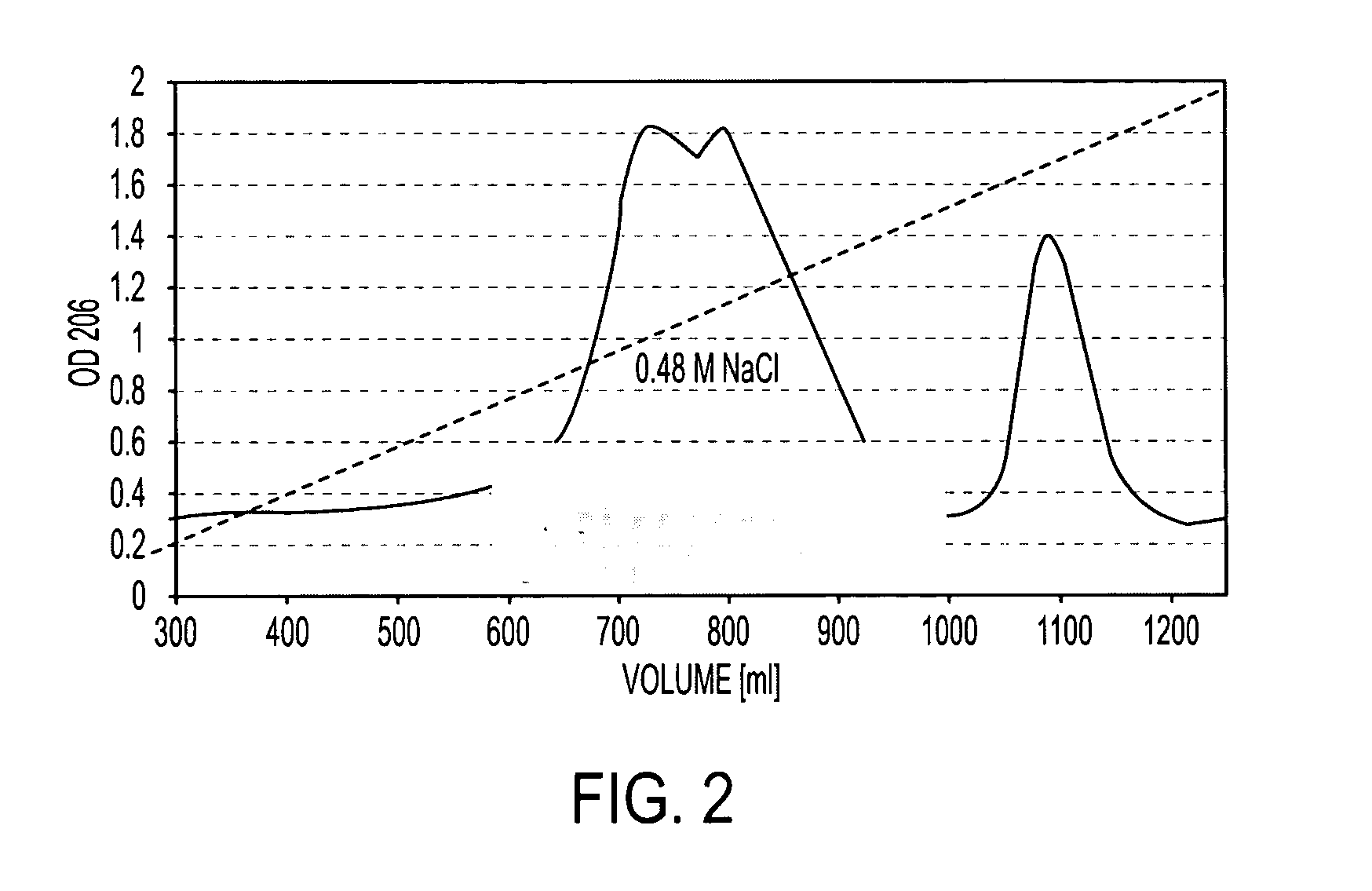
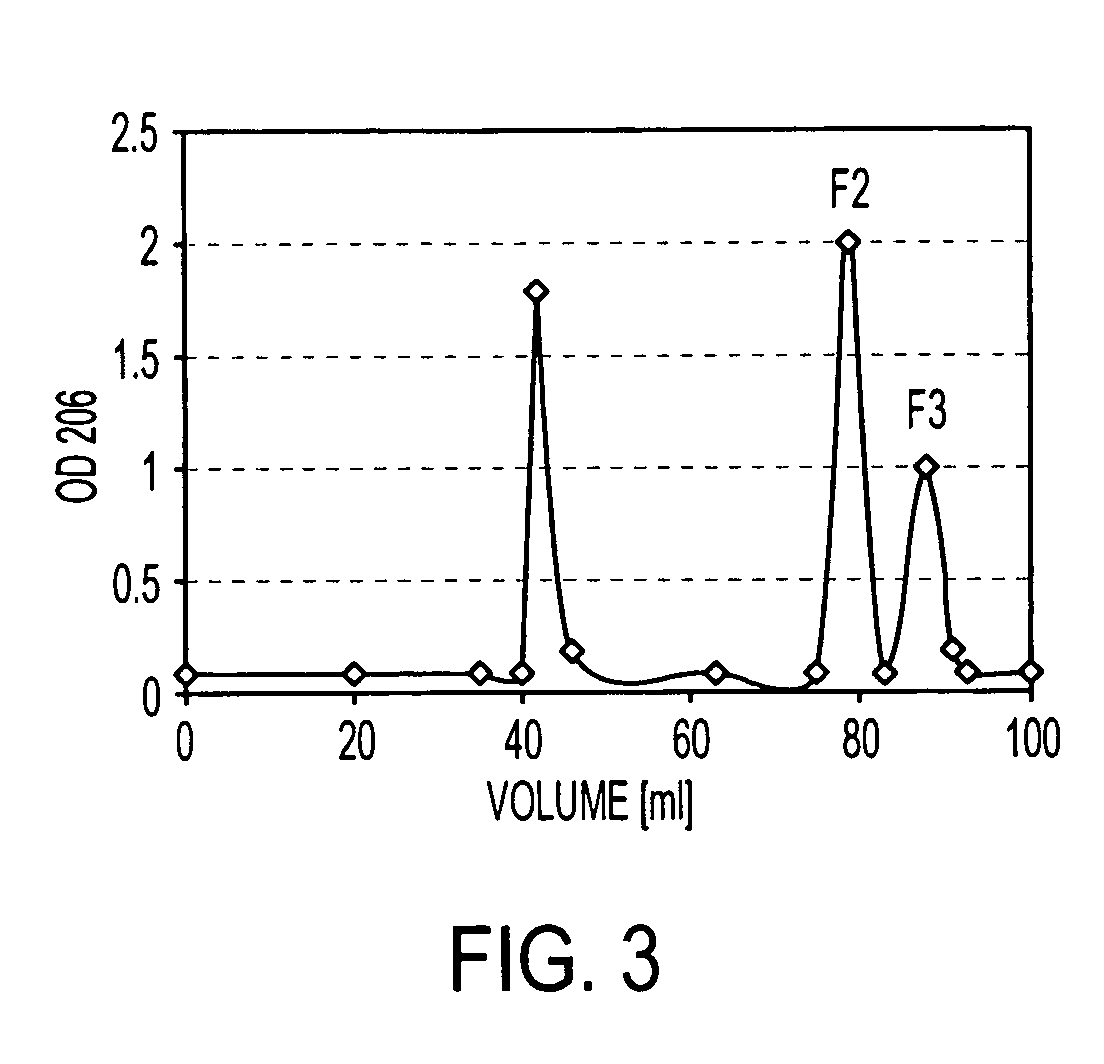
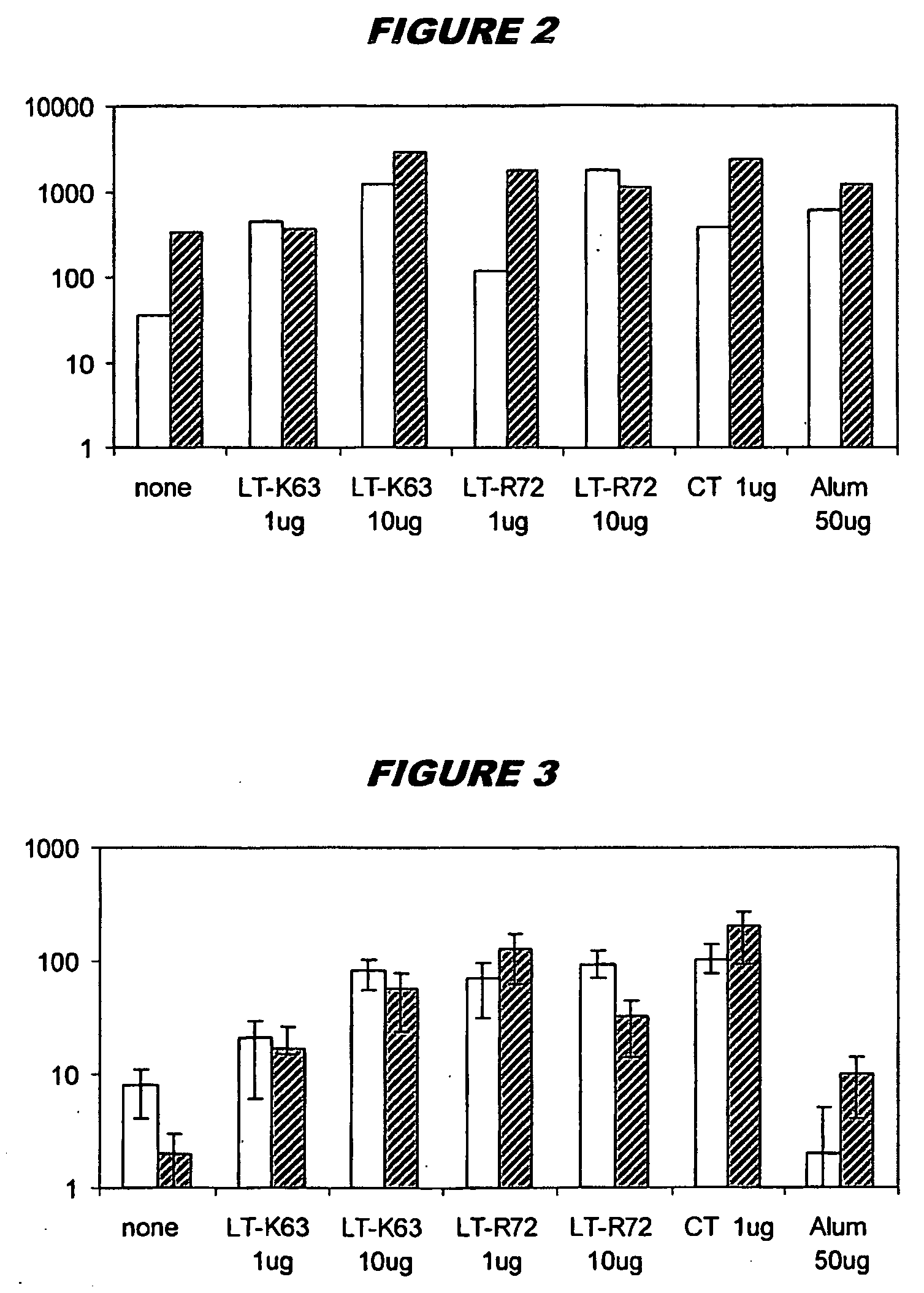
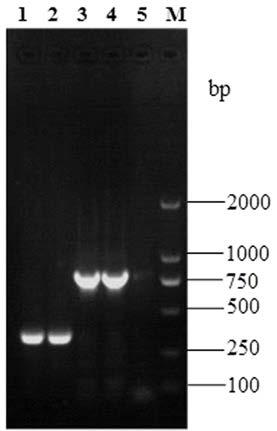
Patents
Literature
123 results about "Haemophilus species" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Kit for quickly detecting 15 pneumonia pathogenic bacteria
ActiveCN107338315AMicrobiological testing/measurementMicroorganism based processesBacteroidesStaphylococcus aureus
The invention discloses a kit for quickly detecting 15 pneumonia pathogenic bacteria. The kit can detect streptococcus pneumoniae, staphylococcus aureus, haemophilus influenzae, mycoplasma pneumoniae, pseudomonas aeruginosa, baumanii, enterococcus faecalis, enterococcus faecium, klebsiella pneumoniae, escherichia coli, enterobacter cloacae, stenotrophomonas maltophilia, burkholderia cepacia, legionella pneumophila and chlamydia pneumoniae which cover clinically common pneumonia pathogenic bacteria difficult to culture. 16S rDNA and specific gene sequences corresponding to the pneumonia pathogenic bacteria are detected by combining gene chips with multiple asymmetric PCR reactions, and the categories of the bacteria in a to-be-detected sample are identified in genus and species. The kit makes up for the defect that current clinical detection of pneumonia pathogenic bacteria is not in time or comprehensive and a novel detection means for early diagnosis and early treatment of patients suffering from pneumonia is provided.
Owner:GENERAL HOSPITAL OF PLA +1
Genes of an otitis media isolate of haemophilus influenzae
InactiveUS20050221439A1Good linkageEasy to modifyAntibacterial agentsSenses disorderStainingMiddle ear
The invention relates to the polynucleotide sequence of a nontypeable stain of Haemophilus influenzae (NTHi) and polypeptides encoded by the polynucleotides and uses thereof. The invention also relates to NTHi genes which are upregulated during or in response to NTHi infection of the middle ear and / or the nasopharynx.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA +1
Genes of an otitis media isolate of nontypeable haemophilus influenzae
ActiveUS20130078254A1Good linkageEasy to modifyAntibacterial agentsSenses disorderStainingMiddle ear
The invention relates to the polynucleotide sequence of a nontypeable stain of Haemophilus influenzae (NTHi) and polypeptides encoded by the polynucleotides and uses thereof. The invention also relates to NTHi genes which are upregulated during or in response to NTHi infection of the middle ear and / or the nasopharynx.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA +1
Haemophilus parasuis LC strain and application thereof
ActiveCN102399724AStrong pathogenicityImproving immunogenicityAntibacterial agentsBacteriaHeterologousDisease
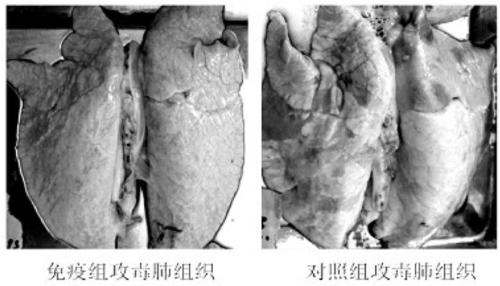
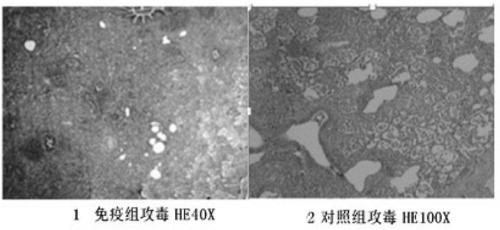
The invention relates to the field of haemophilus parasuis vaccines in veterinary biological products, in particular to a haemophilus parasuis LC strain. The collection number of the strain is CGMCC (China General Microbiological Culture Collection Center) No.5257. The invention also relates to application of the haemophilus parasuis LC strain to preparation of haemophilus parasuis inactivated vaccines. The haemophilus parasuis LC strain has stronger pathogenicity to pigs and has better immunogenicity; an inactivated alumina gel vaccine prepared by the strain is safe and reliable; not only a homologous attacking protection is provided, but also a better cross protection to blood serums type 4, type 5, type 10, type 12, type 14 and type 15 HPS (Hantavirus Pulmonary Syndrome) heterologous attacking can be provided; after the pigs are immunized, a stronger immunity can be generated and the morbidity and the mortality of the inoculated pigs are obviously reduced; the immune effect achieves or is better than the traditional commercialized vaccines in the market; the vaccine has the advantages to compete with like products at home and abroad and is capable of effectively preventing the epidemic and the transmission of a haemophilus parasuis disease and reducing the economic losses caused by the disease, so that the application range is wide.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
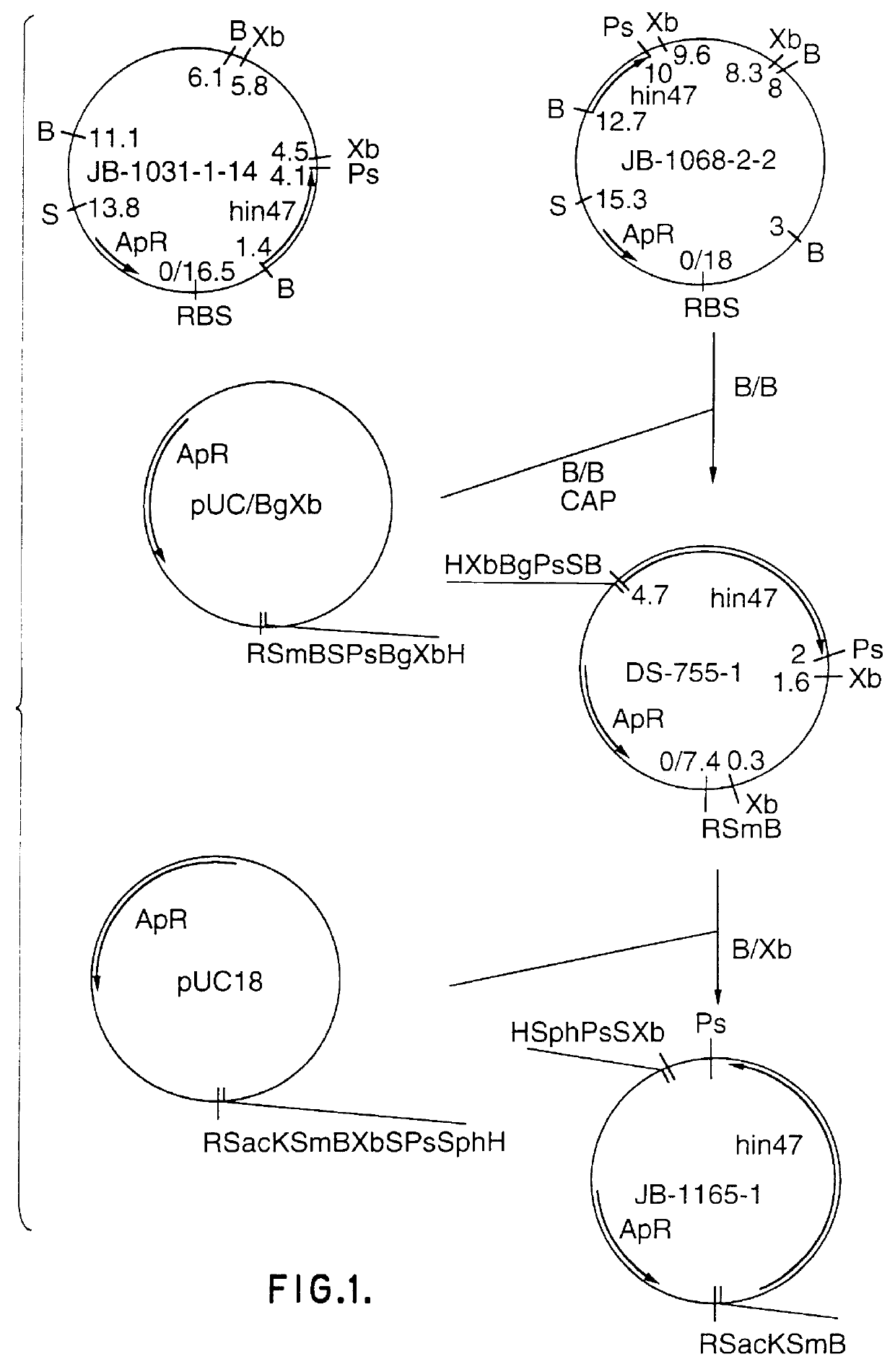
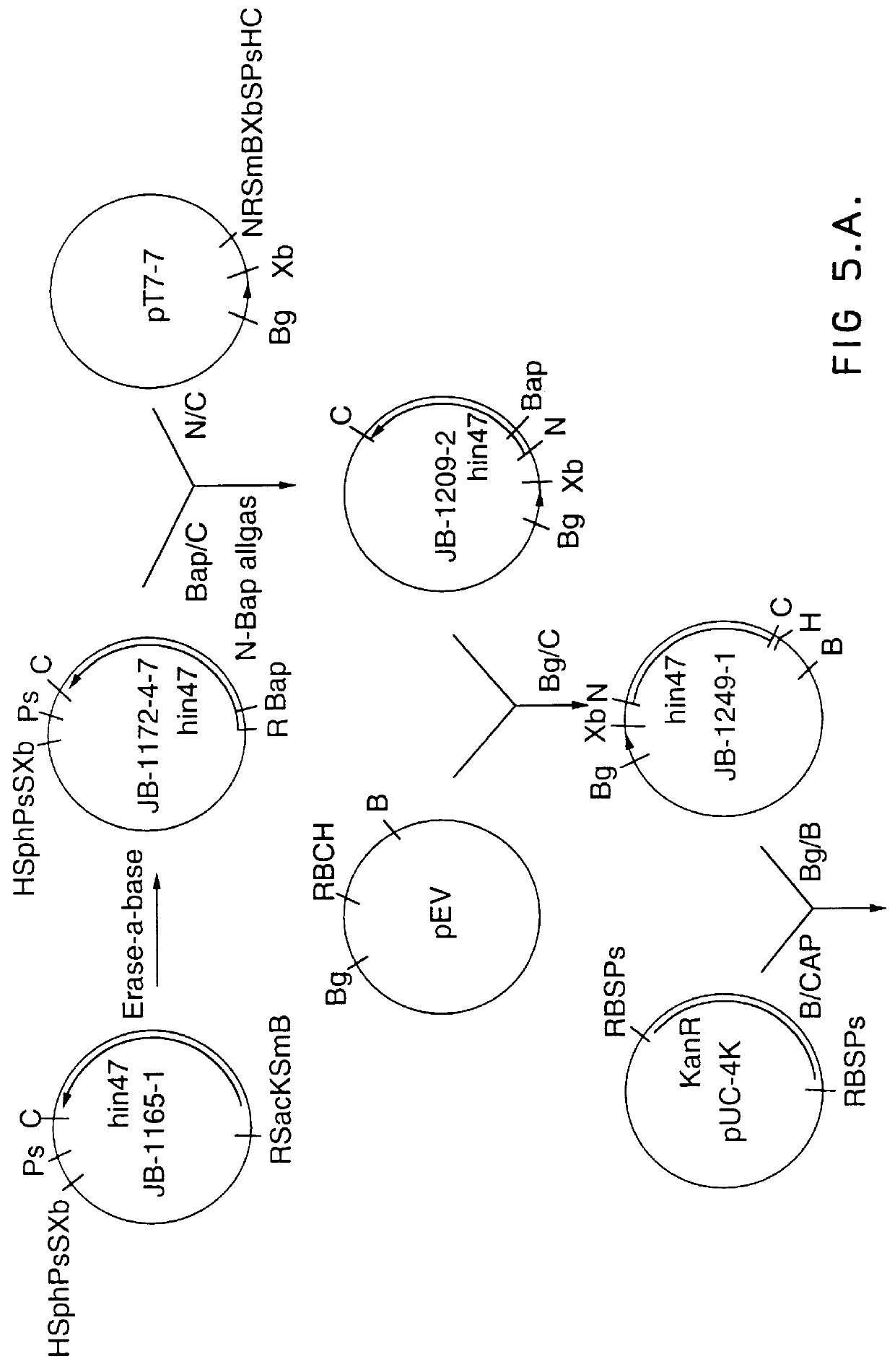
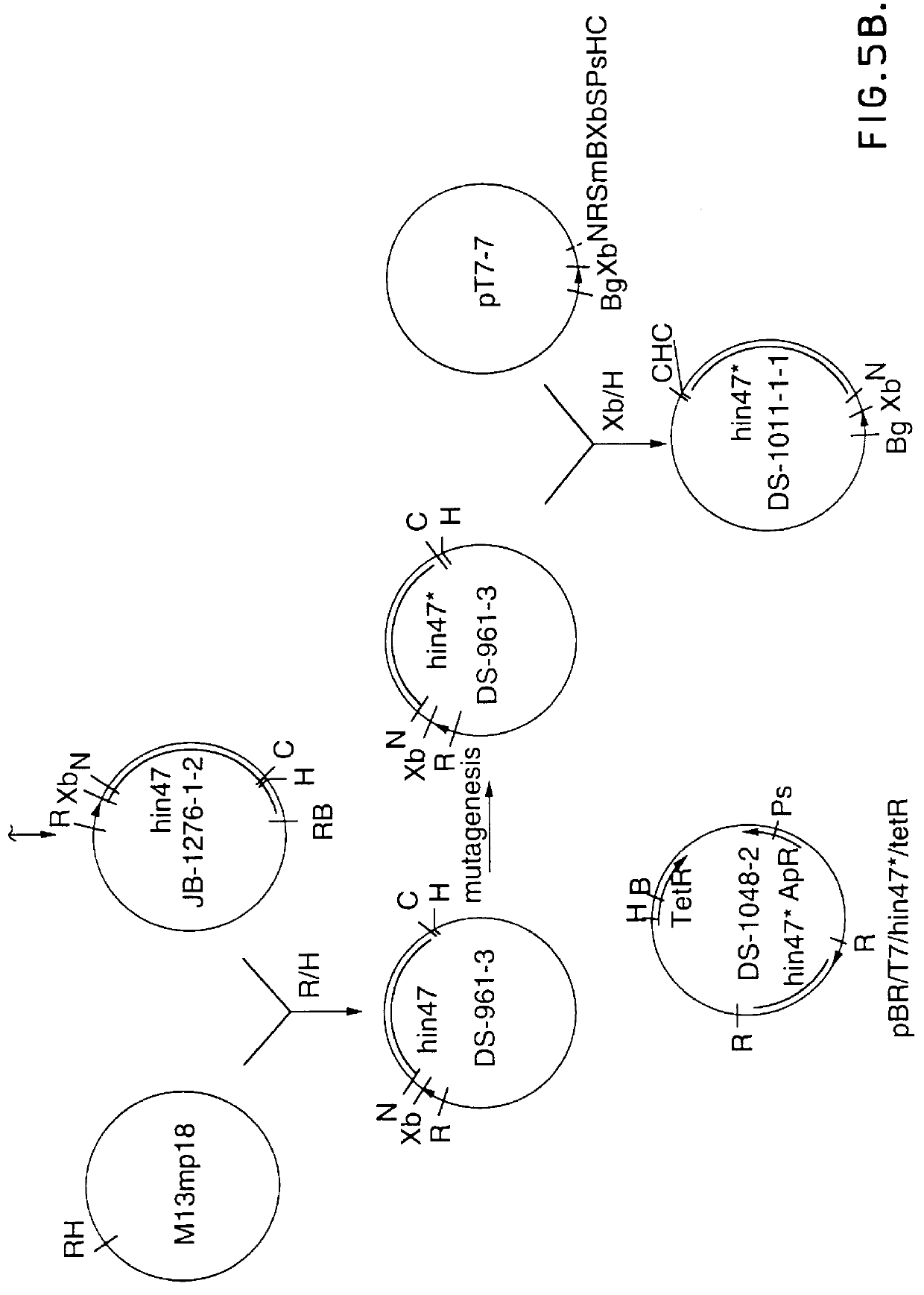
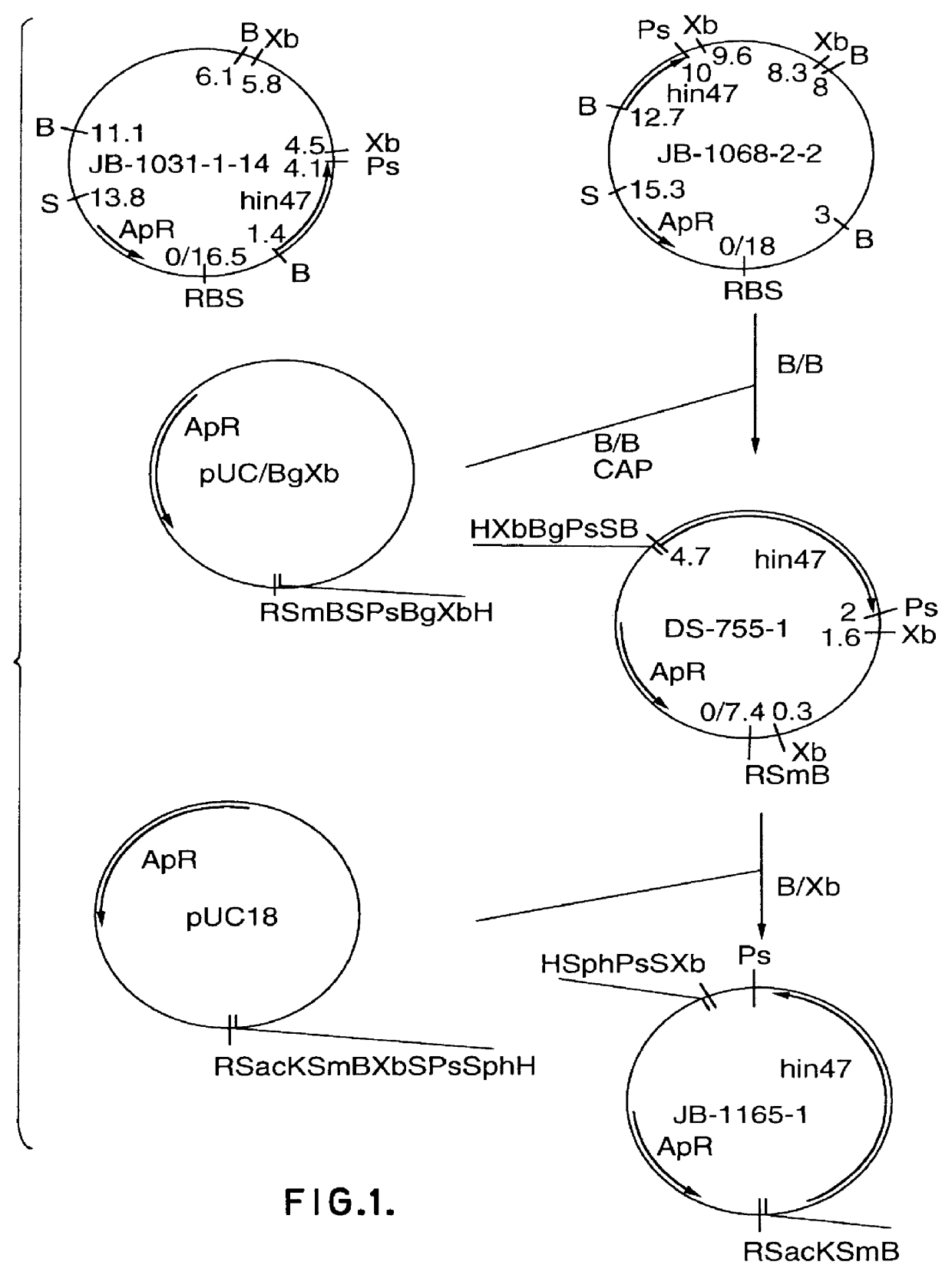
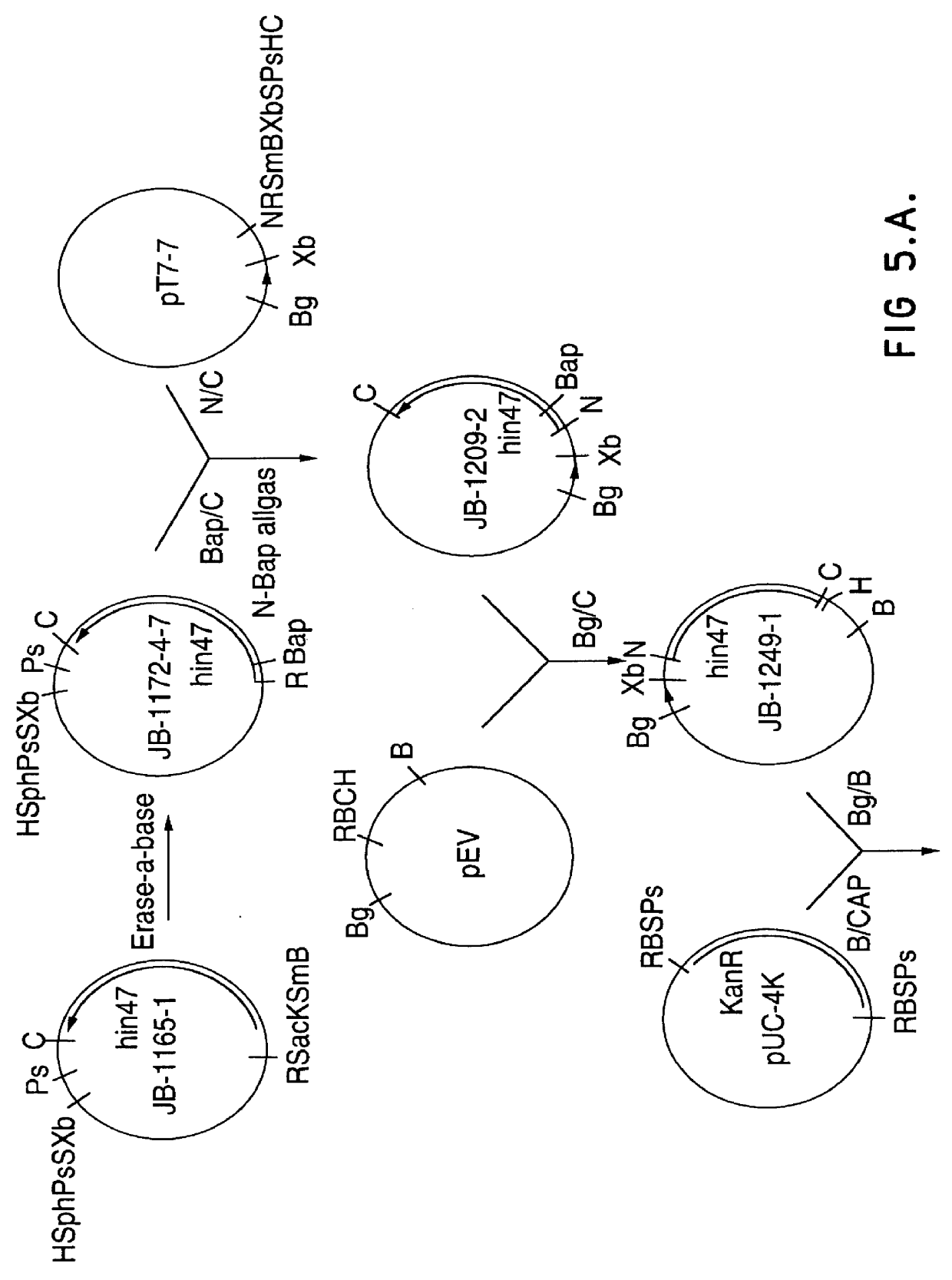
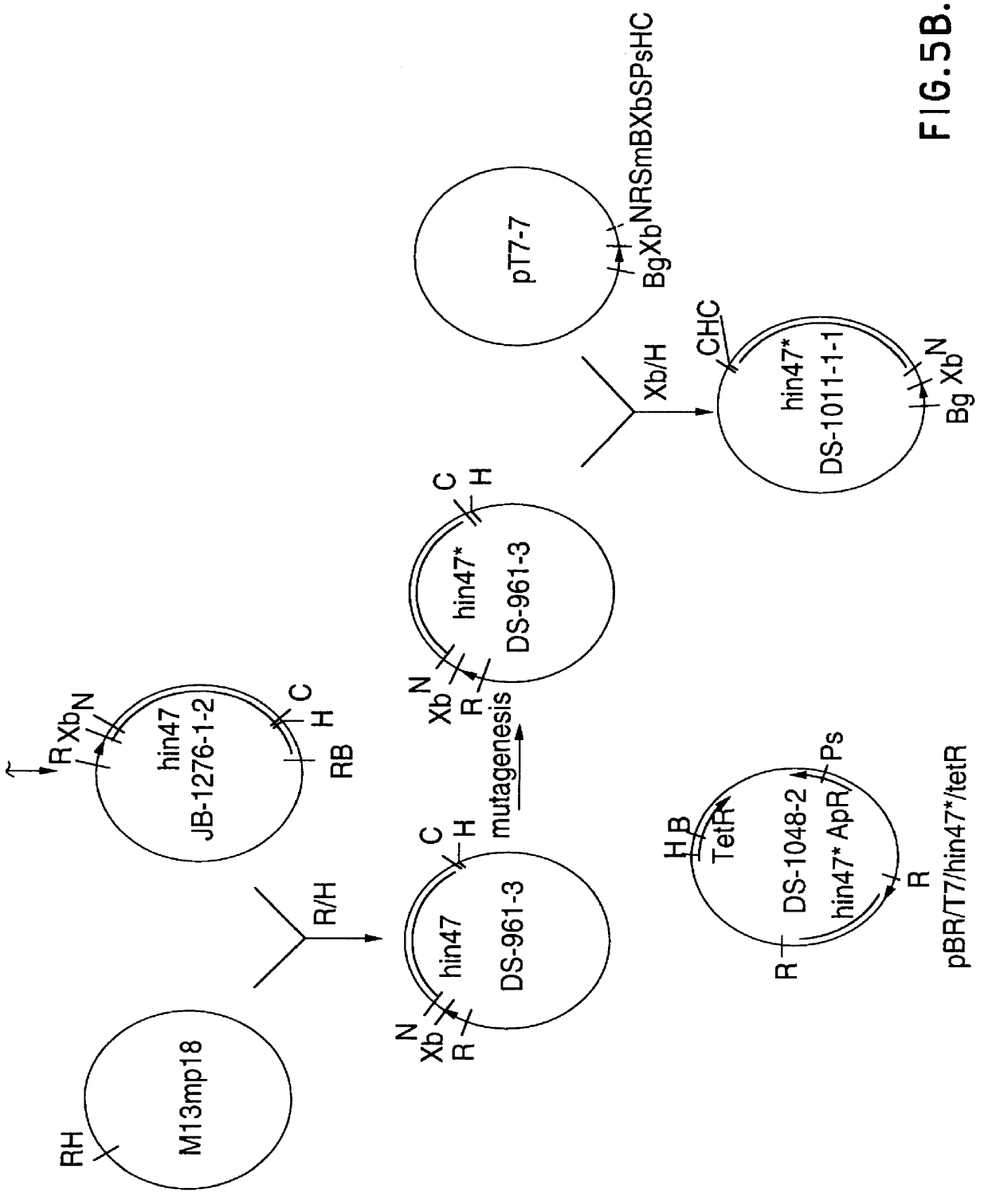
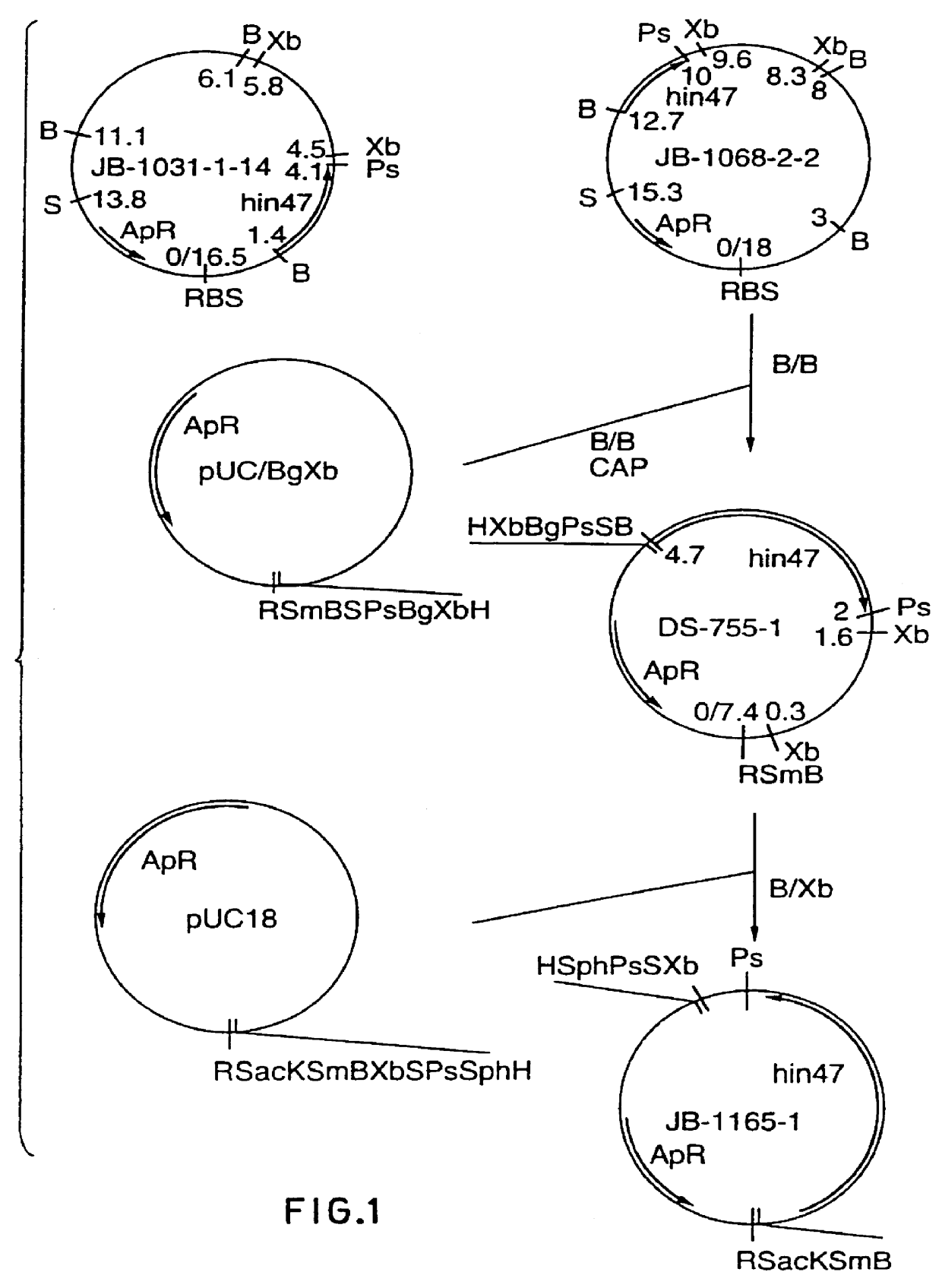
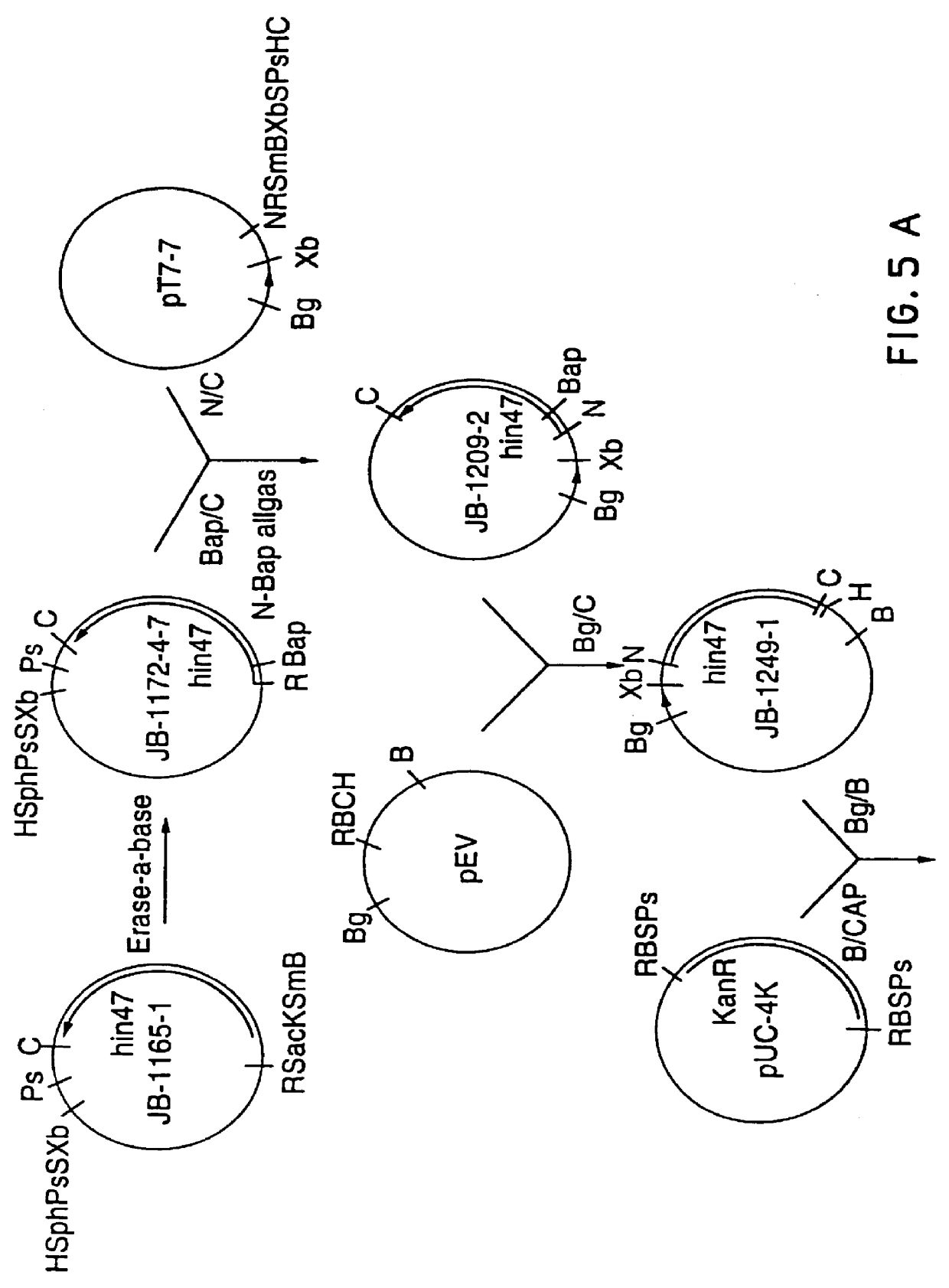
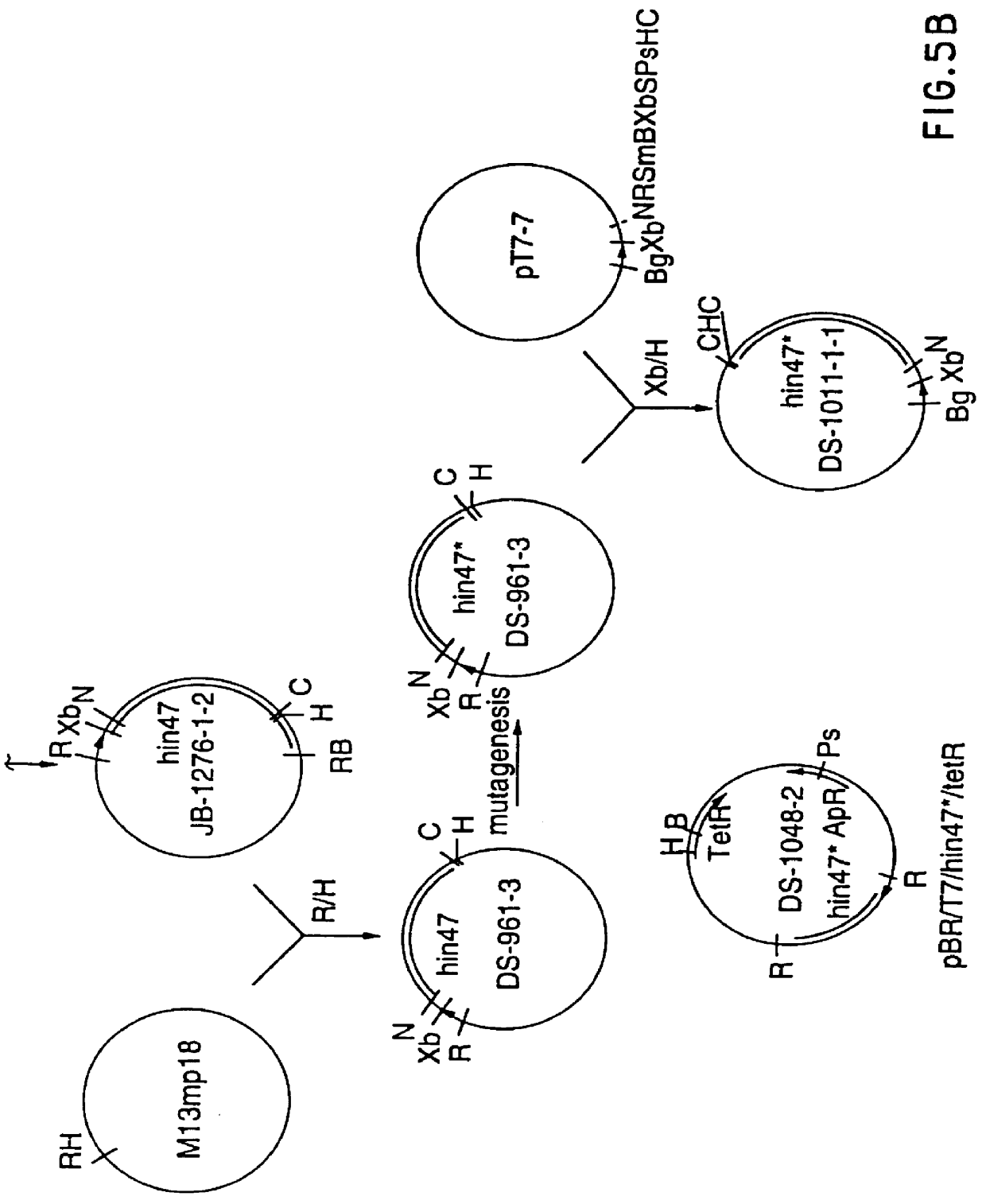
Analog of Haemophilus Hin47 with reduced protease activity
An isolated and purified analog of Haemophilus influenza Hin47 protein has a decreased protease activity which is less than about 10% of that of natural Hin47 protein and preferably substantially the same immunogenic properties as natural Hin47 protein. An isolated and purified nucleic acid molecule encoding the Hin47 analog may be provided in a recombinant plasmid which may be introduced into a cell which is grown to produce the Hin47 analog. Immunogenic compositions comprising the Hin47 analog and the encoding nucleic acid may be formulated as vaccines for in vivo administration to a host, including a human, to confer protection against diseases caused by a bacterial pathogen, including Haemophilus species, such as Haemophilus influenzae, that produces Hin47 protein or a protein capable of inducing antibodies in the host specifically reactive with Hin47 protein. The Hin47 analog and the encoding nucleic acid also may be employed in diagnostic applications.
Owner:CONNAUGHT LAB
Analog of Haemophilus Hin47 with reduced protease activity
An isolated and purified analog of Haemophilus influenzae Hin47 protein has a decreased protease activity which is less than about 10% of that of natural Hin47 protein and preferably substantially the same immunogenic properties as natural Hin47 protein. An isolated an purified nucleic acid molecule encoding the Hin47 analog may be provided in a recombinant plasmid which may be introduced into a cell which is grown to produce the Hin47 analog. Immunogenic compositions comprising the Hin47 analog and the encoding nucleic acid may be formulated as vaccines for in vivo administration to a host, including a human, to confer protection against diseases caused by a bacterial pathogen, including Haemophilus species, such as Haemophilus influenzae, that produces Hin47 protein or a protein capable of inducing antibodies in the host specifically reactive with Hin47 protein. The Hin47 analog and the encoding nucleic acid also may be employed in diagnostic applications.
Owner:CONNAUGHT LAB
Analog of haemophilus Hin47 with reduced protease activity
InactiveUS6020183AProvide protectionImprove efficiencyBacteriaPeptide/protein ingredientsDiseaseBacteroides
An isolated and purified analog of Haemophilus influenzae Hin47 protein has a decreased protease activity which is less than about 10% of that of natural Hin47 protein and preferably substantially the same immunogenic properties as natural Hin47 protein. An isolated an purified nucleic acid molecule encoding the Hin47 analog may be provided in a recombinant plasmid which may be introduced into a cell which is grown to produce the Hin47 analog. Immunogenic compositions comprising the Hin47 analog and the encoding nucleic acid may be formulated as vaccines for in vivo administration to a host, including a human, to confer protection against diseases caused by a bacterial pathogen, including Haemophilus species, such as Haemophilus influenzae, that produces Hin47 protein or a protein capable of inducing antibodies in the host specifically reactive with Hin47 protein. The Hin47 analog and the encoding nucleic acid also may be employed in diagnostic applications.
Owner:CONNAUGHT LAB
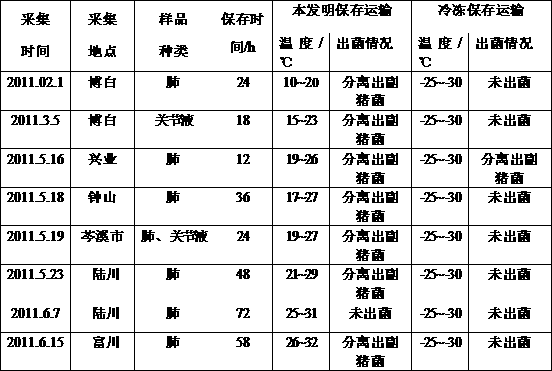
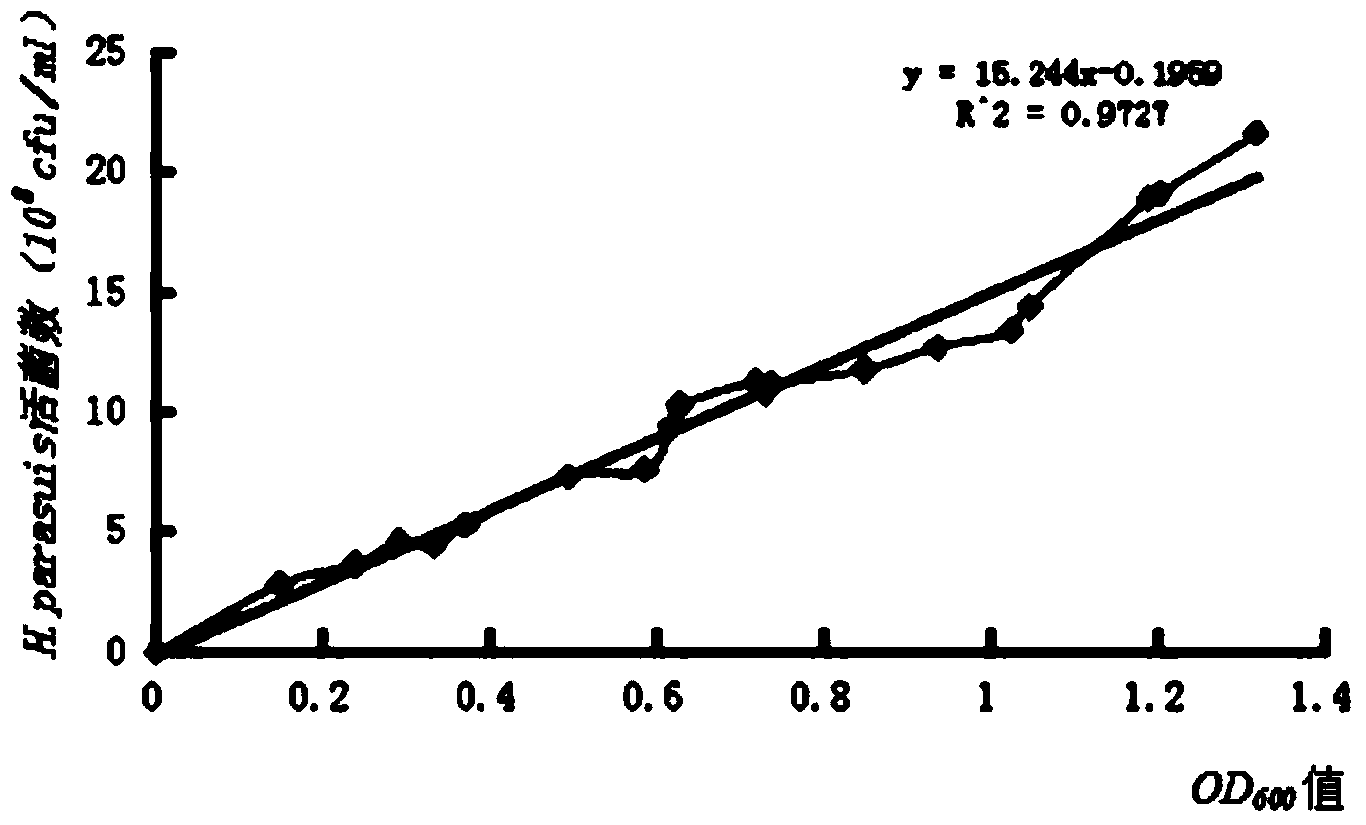
Haemophilus parasuis culture medium
ActiveCN103215208AMaintain integrityIncrease success rateBacteriaMicroorganism based processesSerum igeHaemophilus
The invention discloses a haemophilus parasuis culture medium. A preparation method of the haemophilus parasuis culture medium comprises the steps of preparing a basic culture solution, treating coenzyme A, and perfecting a culture medium, wherein the basic culture solution comprises peptone, tryptone, sodium chloride, dextrose, yeast extract, glycerin and distilled water, and the basic culture solution, the coenzyme A and fetal bovine serum are uniformly mixed so as to obtain the haemophilus parasuis culture medium. The haemophilus parasuis culture medium provided by the invention can keep haemophilus parasuis, thereby facilitating the transportation of suspicious haemophilus parasuis samples; and the death of haemophilus parasuis is not caused in the process of transportation, thereby increasing the separation probability of the haemophilus parasuis.
Owner:广西悦牧生物科技有限公司
Analog of haemophilus Hin47 with reduced protease activity
InactiveUS6153580AImprove stabilityImprove thermal stabilityAntibacterial agentsBiocideBacteroidesDisease
An isolated and purified analog of Haemophilus influenzae Hin47 protein has a decreased protease activity which is less than about 10% of that of natural Hin47 protein and preferably substantially the same immunogenic properties as natural Hin47 protein. An isolated an purified nucleic acid molecule encoding the Hin47 analog may be provided in a recombinant plasmid which may be introduced into a cell which is grown to produce the Hin47 analog. Immunogenic compositions comprising the Hin47 analog and the encoding nucleic acid may be formulated as vaccines for in vivo administration to a host, including a human, to confer protection against diseases caused by a bacterial pathogen, including Haemophilus species, such as Haemophilus influenzae, that produces Hin47 protein or a protein capable of inducing antibodies in the host specifically reactive with Hin47 protein. The Hin47 analog and the encoding nucleic acid also may be employed in diagnostic applications.
Owner:LOOSMORE SHEENA M +4
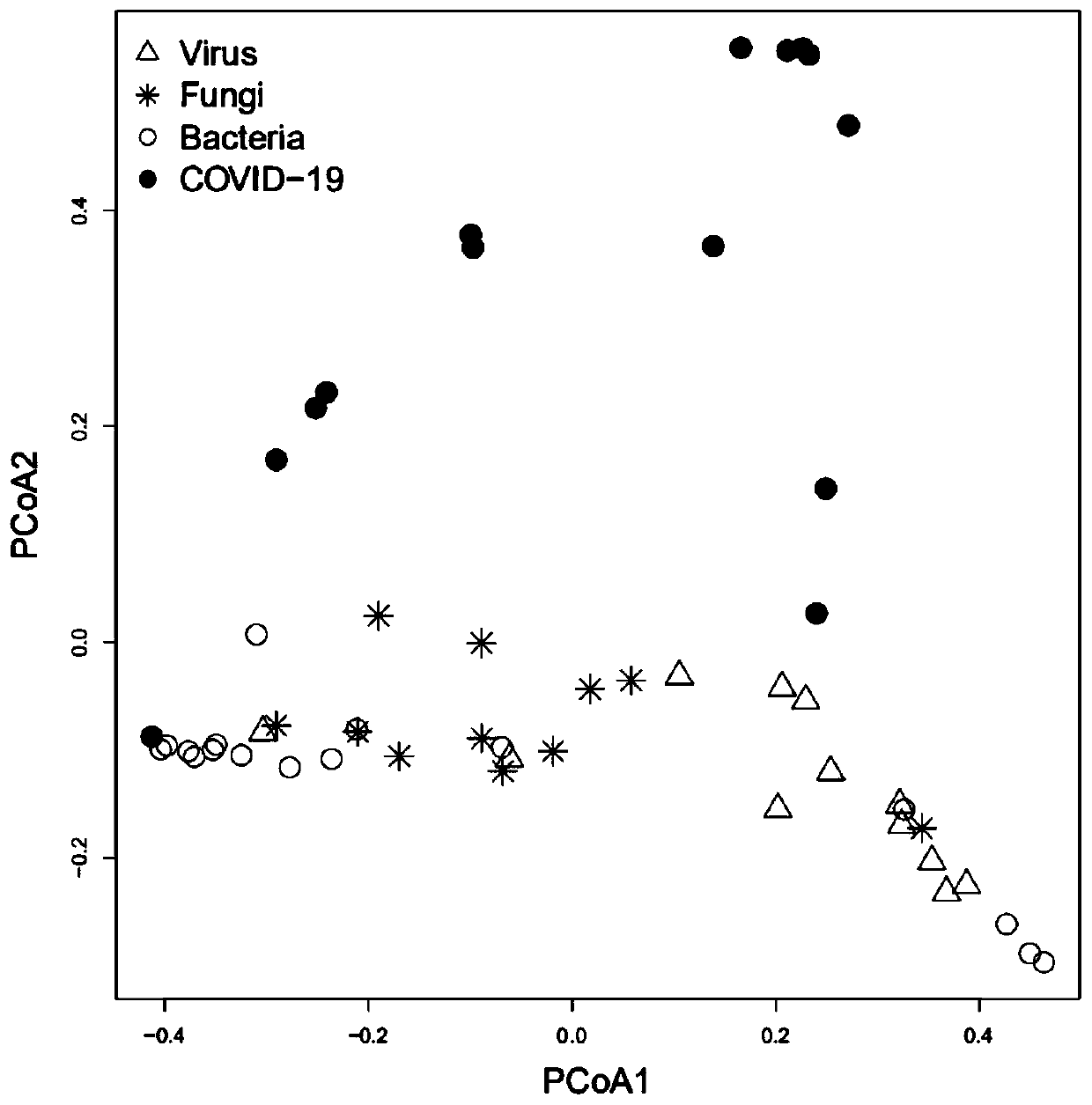
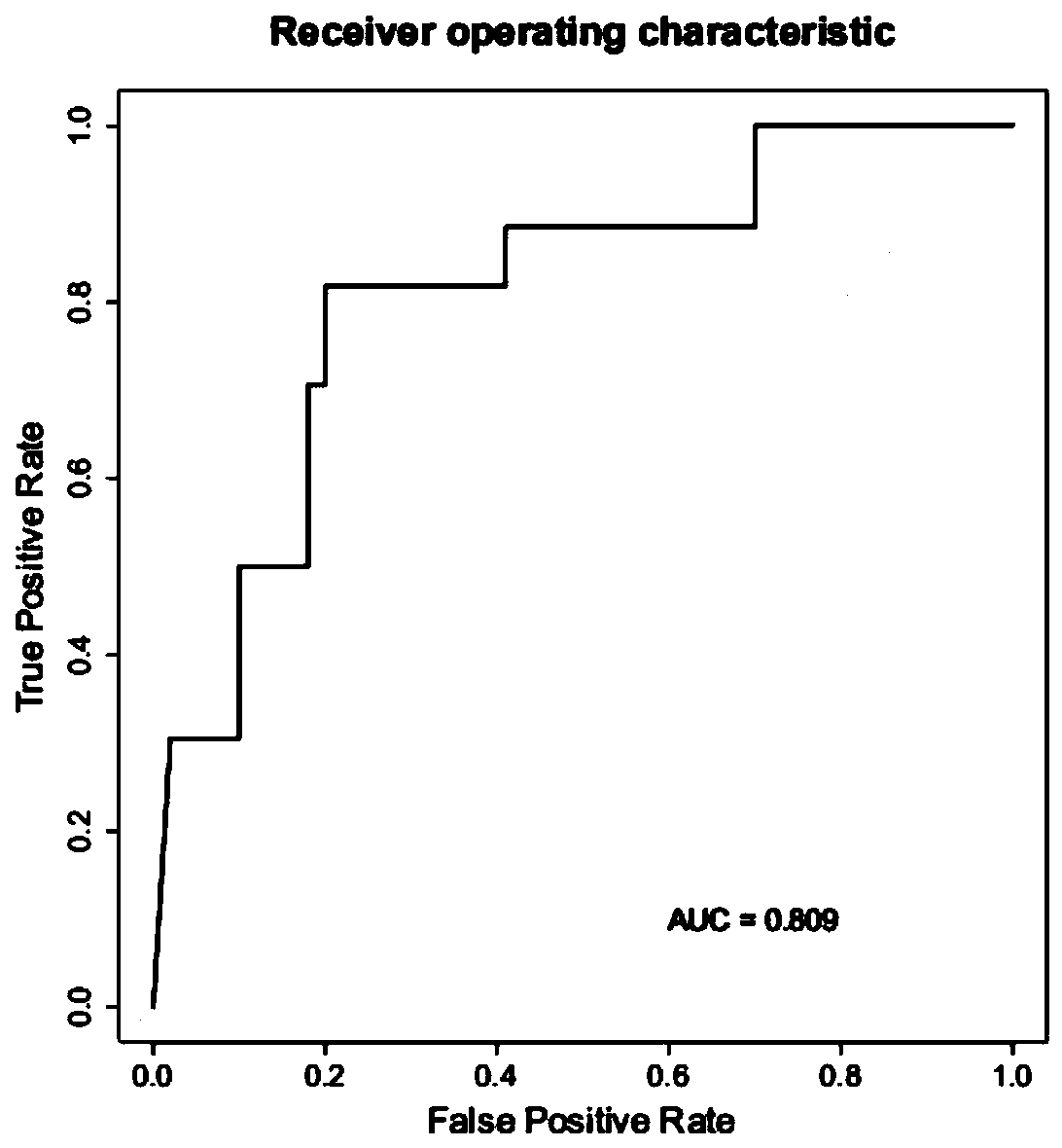
Strain marker for assisting COVID-19 diagnosis and application thereof
ActiveCN111378788AImprove diagnostic capabilitiesMake up for the defect of false negative testMicrobiological testing/measurementAgainst vector-borne diseasesPrevotella nigrescensAggregatibacter
The invention relates to a strain marker for assisting COVID-19 diagnosis and application thereof and belongs to the technical field of pathogenic microorganism infection detection. The strain markercomprises at least one of haemophilus parainfluenzae, propionibacterium, veillonella atypica, aggregatibacter segnis, alloprevotellatannerae, campylobacter showae, fusobacteria, fusobacterium nucleatum, fusobacterium periodonticum, gemella sanguinis, haemophilus parahaemolyticus, haemophilus sputorum, porphyromonas gingivalis, prevotella intermedia, prevotella multifurmis, prevotella nanceiensis,prevotella nigrescens and prevotella timonensis. A prediction model ROC obtained by the strain marker has an AUC value being 0.910, and the strain marker can be used for assisting diagnosis of SARS-CoV-2 infection, so that the diagnosing performance on COVID-19 pneumonia can be improved, and the defect that viral nucleic acid detection is false negative can be overcome to a certain extent.
Owner:广州微远医疗器械有限公司 +4
High molecular weight surface proteins of non-typeable haemphilus
High molecular weight surface proteins of non-typeable Haemophilus influenzae which exhibit immunogenic properties and genes encoding the same are described. Specifically, genes coding for two immunodominant high molecular weight proteins, HMW1 and HMW2, have been cloned, expressed and sequenced, while genes coding for high molecular proteins HMW3 and HMW4 have also been cloned, expressed and sequenced.
Owner:BARENKAMP STEPHEN J
Multiple quantitative PCR (polymerase chain reaction) kit for quick combined detection of four bacteria difficult to cultivate and identify
ActiveCN105063218ARapid identificationIdentification is simple and effectiveMicrobiological testing/measurementMicroorganism based processesMoraxella catarrhalisBacilli
The invention provides a multiple quantitative PCR (polymerase chain reaction) kit for quick combined detection of four bacteria difficult to cultivate and identify. The multiple quantitative PCR kit comprises four PCR reaction systems, wherein the four PCR reaction systems comprise AllGlo fluorescence probes and forward and reverse primers aimed at the following four pathogens which cause child bacterial pneumonia and are clinically difficult to cultivate and identify: haemophilus influenza, streptococcus pneumonia, moraxella catarrhalis and legionella pneumophila. According to the multiple quantitative PCR kit, the design is reasonable, the infection of the four pathogens, clinically difficult to cultivate and identify, of child bacterial pneumonia can be easily, conveniently, quickly and parallelly detected in a reaction tube at the same time, the situation that four bacteria are detected at the same time through single-tube PCR is achieved, quantitative detection is achieved, the kit is easy and quick to operate, high in sensitivity, good in specificity and repeatability, accurate and reliable in result, early specific diagnosis, prevention and treatment can be provided for patients suffering from infantile pneumonia according to the bacterial infection titer, and the kit has great clinical practicability for interdicting an infection source, reducing infection or mixed infection of the four bacteria and monitoring the clinical curative effect.
Owner:HANGZHOU FIRST PEOPLES HOSPITAL
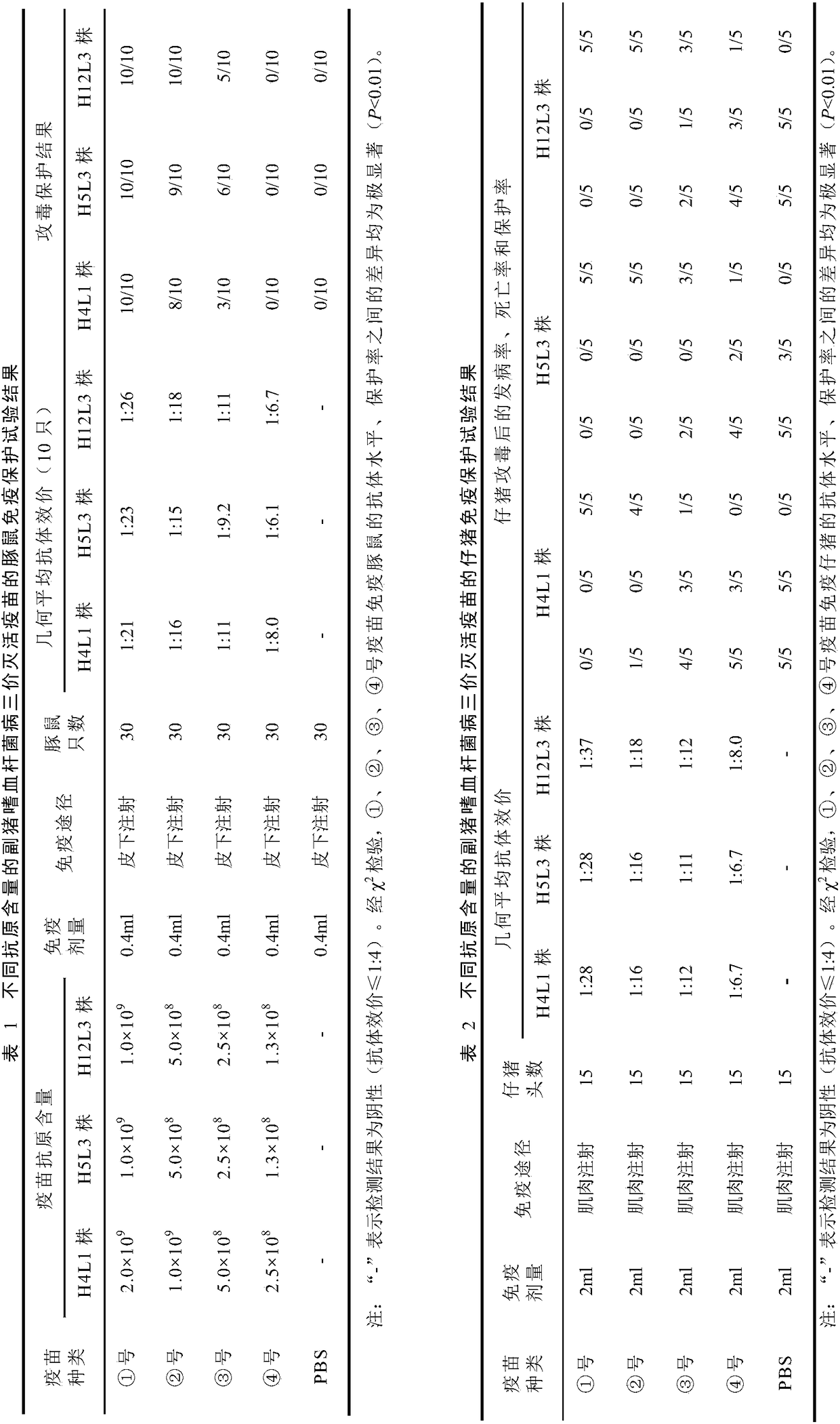
Haemophilus parasuis trivalent inactivated vaccine as well as production method and application thereof
InactiveCN108441446AImprove securityImprove immune efficiencyAntibacterial agentsBacterial antigen ingredientsDiseaseHaemophilus
The invention discloses a haemophilus parasuis trivalent inactivated vaccine as well as a production method and application thereof. The haemophilus parasuis trivalent inactivated vaccine contains a haemophilus parasuis type-4 H4L1 strain, a type-5 H5L3 strain and a type-12 H12L3 strain being inactivated by a formaldehyde solution, as well as a water-based immunologic adjuvant, wherein the haemophilus parasuis type-4 H4L1 strain, the type-5 H5L3 strain and the type-12 H12L3 strain are all collected in the China Center for Type Culture Collection on 11 January, 2018 with the collection numbersof CCTCC M 2018019, CCTCC M 2018020 and CCTCC M 2018021 respectively. The trivalent inactivated vaccine disclosed by the invention is used for preventing the haemophilus parasuis disease caused by thetype-4, type-5 and type-12 haemophilus parasuis, and has the advantages of high security, high immune efficacy, and long immunity period and the like.
Owner:HENAN UNIV OF SCI & TECH +1
Serum 4 type haemophilus parasuis and application thereof
InactiveCN104312964AEffective controlBiological performance is stableAntibacterial agentsBacteriaSerum igePig farms
The invention provides a serum 4 type haemophilus parasuis strain HN1009 which is preserved in CCTCC (China Center For Type Culture Collection) with the preservation number of NO: M20-14126. The strain is stable in biological performance, has relatively strong pathogenicity and is used for grafting piglets after being activated, so that the strain has good immunogenicity. The strain which is used as a monovalent vaccine prepared from a vaccine candidate strain is good in safety, can be used for generating relatively high antibodies to the piglets, is long in duration, can be used for resisting attack of homotype wild strains and has a good immune efficacy. In clinical use, the deactivated vaccine is used for effectively reducing occurrence of haemophilus parasuis so as to reduce the economical loss of a pig farm, so that the immune effect reaches or is superior to existing marketed commercialized vaccines.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT
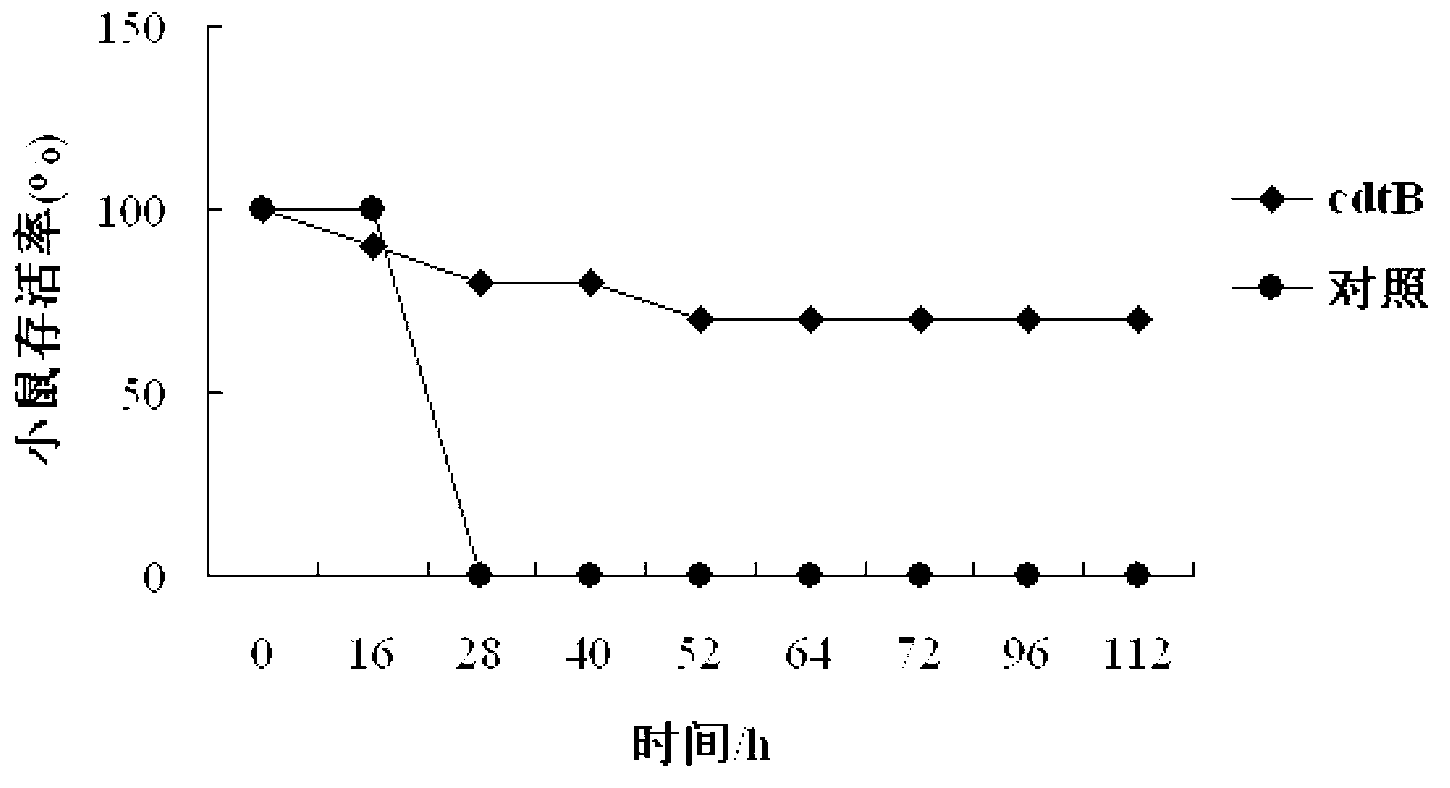
Haemophilus parasuis (Hps) immunoprotecive antigen CdtB
The invention relates to identification of a Haemophilus parasuis (Hps) immunoprotecive antigen, separation and cloning of a protein gene and application of a protein coded by the Hps immunoprotecive antigen in a vaccine. According to the invention, a new protein CdtB having immunogenicity is separated from Hps (the culture collection number is CVCC3361), and the nucleotide sequence is shown as SEQ ID NO:2 in a sequence table and is formed by coding 277 amino acids. The CdtB is a new protein having immunogenicity and can provide effective immunoprotection for Hps infection in mice. The invention also comprises preparation of an Escherichia coli recombinant bacterium BL21 / Hps-CdtB expressing the immunogenicity protein gene CdtB. The recombinant Hps CdtB protein expressed by the invention has favorable safety and protection efficacy, and the immunoprotection effect is up to 70%.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Haemophilus parasuis detection kit and detection method thereof
ActiveCN104711359AHigh sensitivityAccurate distinctionMicrobiological testing/measurementMicroorganism based processesPasteurellaBacilli
The invention relates to a haemophilus parasuis detection kit and a detection method thereof, and belongs to the technical field of molecular biology. The detection kit comprises a primer pair, PCR Mix, a position control and dd H2O. The haemophilus parasuis detection kit disclosed by the invention has the primer pair designed according to an mviN gene sequence in a high conserved domain, is good in specificity, and can accurately distinguish the haemophilus parasuis strain LC from haemophilus paragallinarum, actinobacillus pleuropneumoniae, pasteurella muhocida, arcanobacterium pyogenes, staphylococcus aureus and streptococcus suis; the detection kit and the detection method provided by the invention are high in sensitivity, short in consumed time, accurate in detection, and important in significance of monitoring haemophilus parasuis reproduction, disease occurrence and prevalence as well as timely control of the disease.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Compositions comprising neisseria meningitidis antigens from serogroups b and c
ActiveUS20050074450A1Easy to prepareEasy to manageAntibacterial agentsBacterial antigen ingredientsProtective antigenImmunogenicity
International patent application WO99 / 61053 discloses immunogenic compositions that comprise N. meningitidis serogroup C oligosaccharide conjugated to a carrier, in combination with N. meningitidis serogroup B outer membrane protein. These are disclosed in the present application in combination with further Neisserial proteins and / or protective antigens against other pathogenic organisms (e.g. Haemophilus influenzae, DTP, HBV, etc.).
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Gene chip for identification of seven swine disease pathogens and detection method thereof
InactiveCN105063760AStrong and stable hybridization signalStrong specificityNucleotide librariesMicrobiological testing/measurementSequence analysisDisease
The invention discloses a gene chip for identification of seven swine disease pathogens and a detection method thereof. The gene chip can be used for detection of porcine actinobacillus pleuropneumoniae, haemophilus parasuis, mycoplasma hyopneumoniae, porcine circovirus type 2, porcine reproductive and respiratory syndrome virus, classical swine fever virus and porcine transmissible gastroenteritis virus. A PCR primer is designed by means of standard strain genome sequence analysis, cloning and sequencing analysis are carried out on a target gene, and specific probes are designed to construct the gene chip for detection, and optimization is performed to obtain a detection system. The invention aims to establish a method with the advantages of high sensitivity, strong specificity, time saving and labor saving, and easy observation of results to detect the seven important swine disease pathogens.
Owner:INSPECTION & QUARANTINE TECH CENT OF CHONGQING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Haemophilus parasuis outer membrane protein P5 (OMP5) resistant monoclonal antibody, hybridoma cell strain and application
InactiveCN102876635ANo cross reactionStrong specificityImmunoglobulins against bacteriaMicroorganism based processesEscherichia coliBordetella
The invention discloses a haemophilus parasuis outer membrane protein P5 (OMP5) resistant monoclonal antibody, a hybridoma cell strain and an application. The hybridoma cell strain is preserved in the China center for type culture collection (CCTCC), and the preservation serial number is CCTCCC2012135. The monoclonal antibody prepared by the hybridoma cell strain is good in specificity, high in valence, high in generality, free from cross reaction with swine Escherichia coli, swine pasteurella, swine pleuropneumonia actinobacillus, streptococcus suis and swine bordetella bacilli, capable of detecting haemophilus parasuis with different serotypes and widely applicable to etiology diagnosis, serology detection and immunology detection and prevention of haemophilus parasuis diseases, and the enzyme-linked immuno sorbent assay (ELISA) antibody valence can reach 1:204800 after purification.
Owner:广东省农业科学院兽医研究所
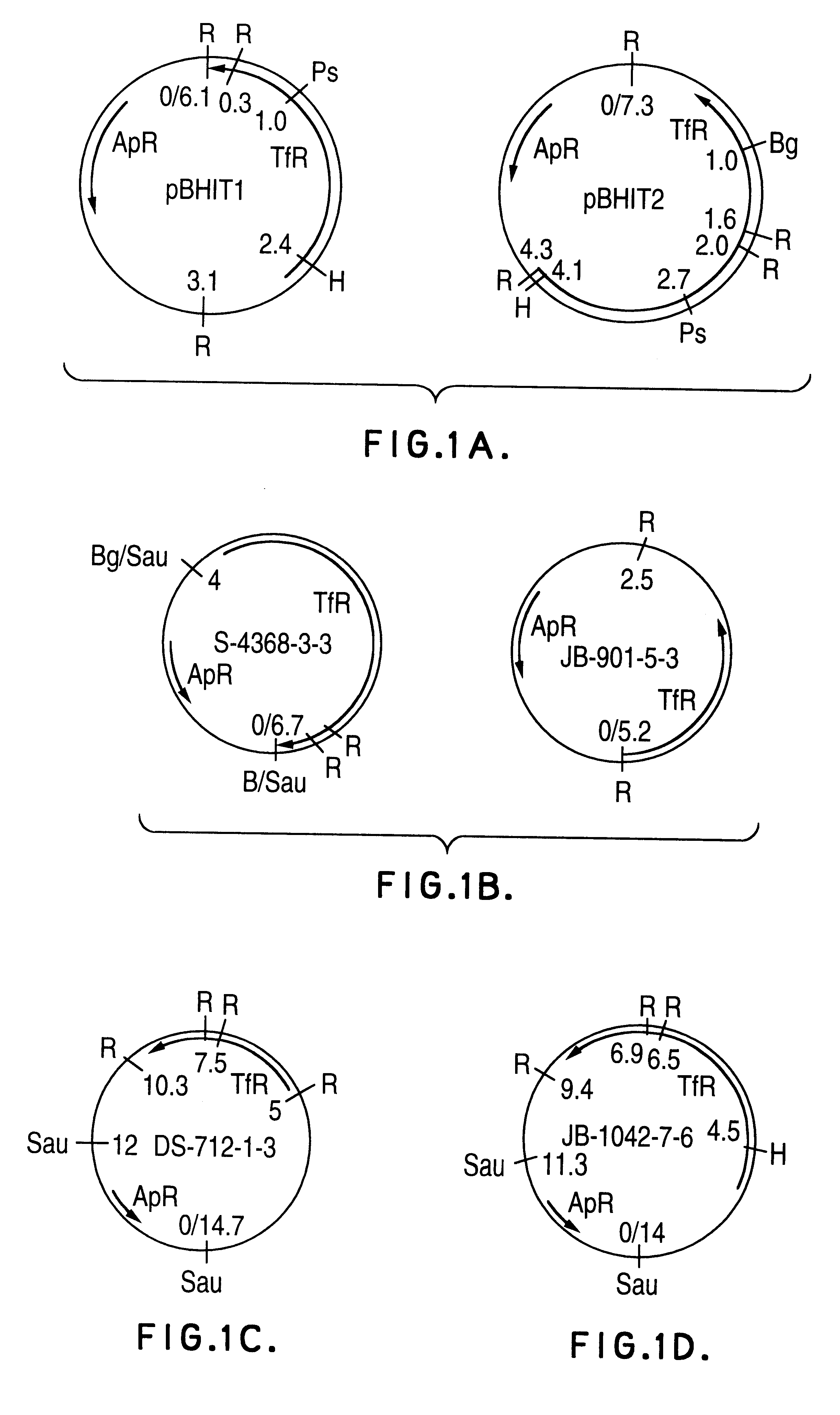
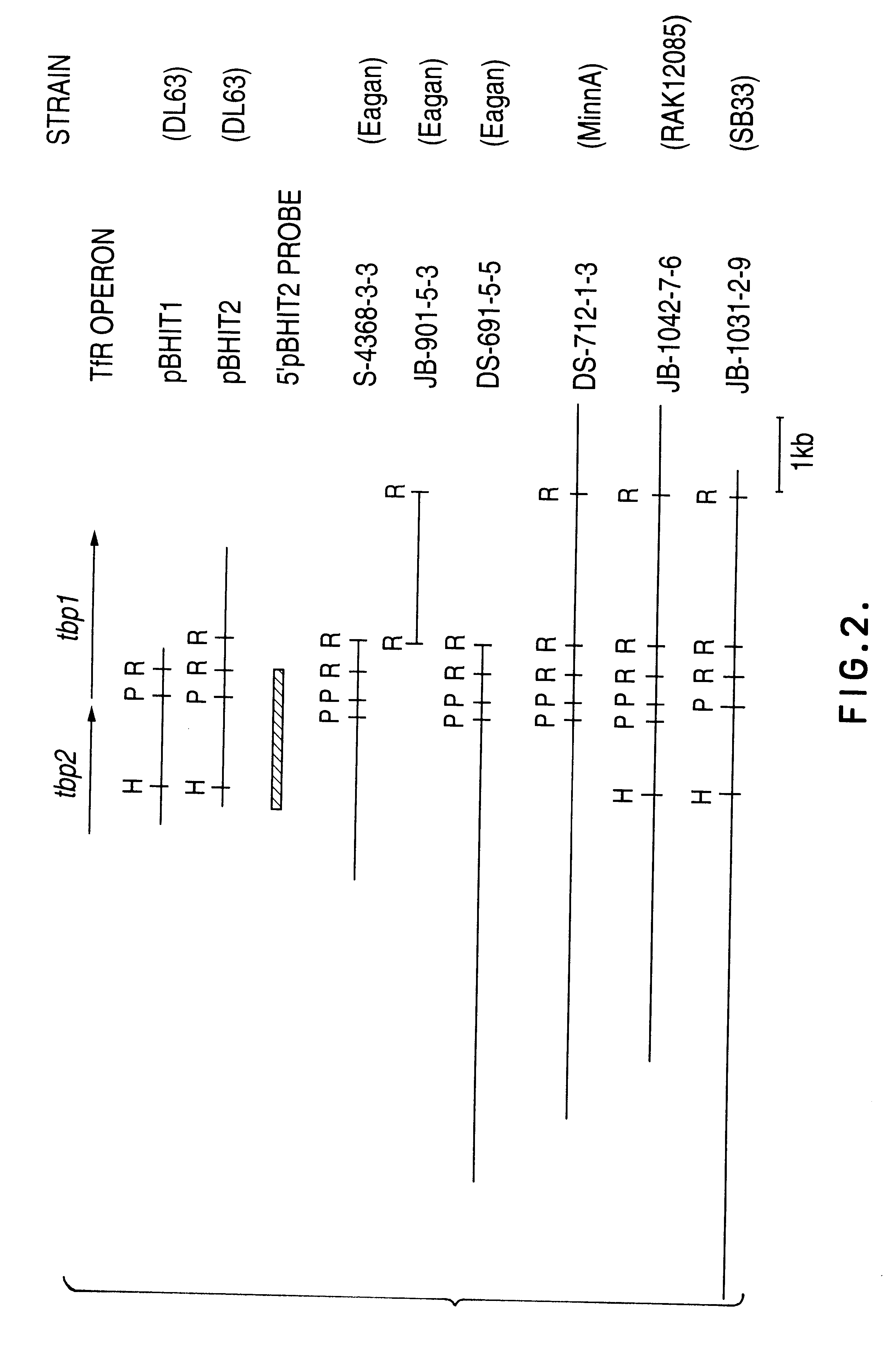
Transferrin receptor genes
Purified and isolated nucleic acid is provided which encodes a transferrin receptor protein of a strain of Haemophilus or a fragment or an analog of the transferrin receptor protein. The nucleic acid sequence may be used to produce peptides free of contaminants derived from bacteria normally containing the Tbp1 or Tbp2 proteins for purposes of diagnostics and medical treatment. Furthermore, the nucleic acid molecule may be used in the diagnosis of infection. Also provided are recombinant Tbp1 or Tbp2 and methods for purification of the same. Live vectors expressing epitopes of transferrin receptor protein for vaccination are provided.
Owner:AVENTIS PASTEUR LTD
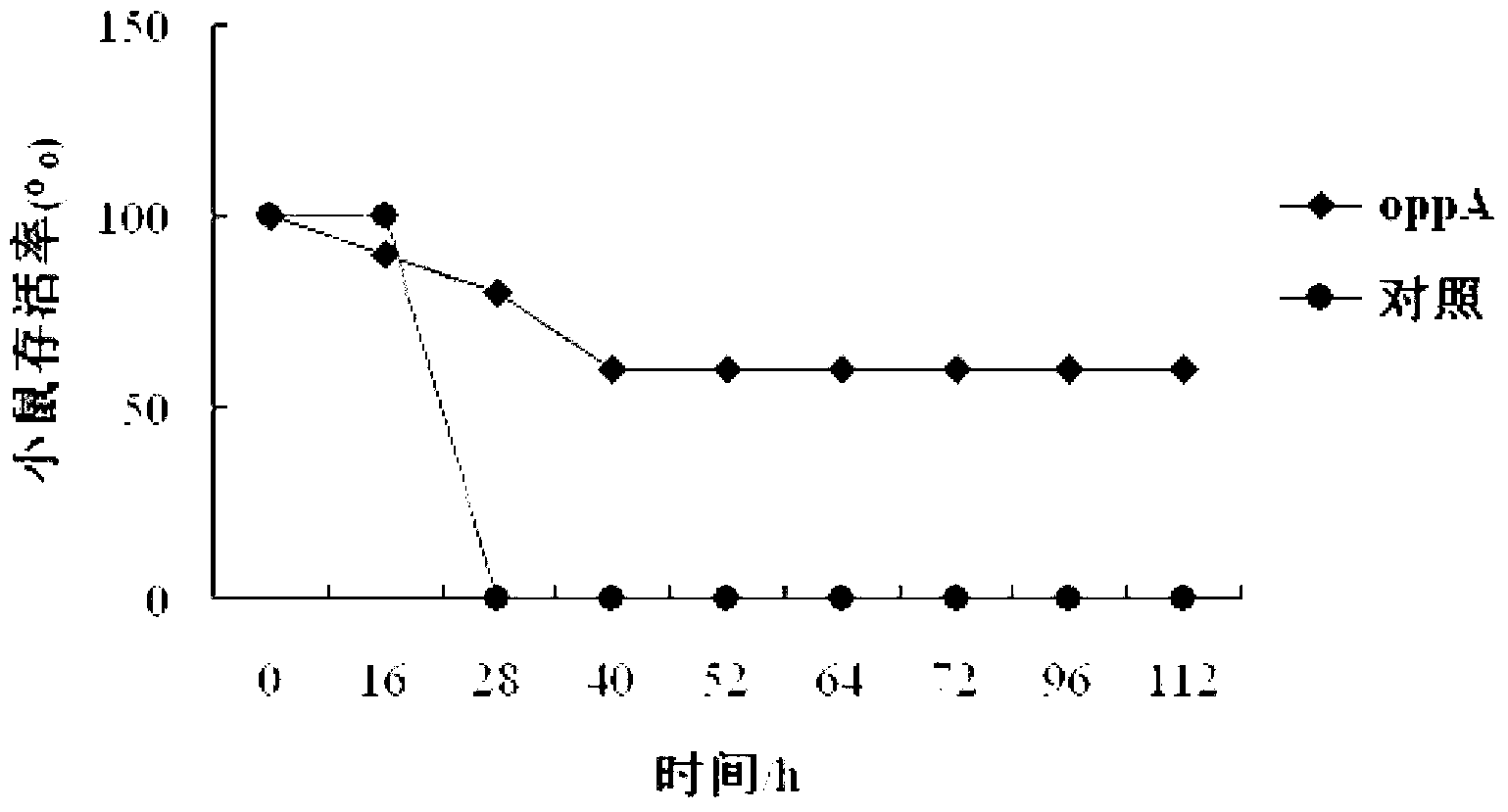
Haemophilus parasuis (Hps) immunoprotecive antigen OppA
The invention relates to identification of a Haemophilus parasuis (Hps) immunoprotecive antigen, separation and cloning of a protein gene and application of a protein coded by the Hps immunoprotecive antigen in a vaccine. According to the invention, a new protein OppA having immunogenicity is separated from Hps (the culture collection number is CVCC3361), and the nucleotide sequence is shown as SEQ ID NO:2 in a sequence table and is formed by coding 545 amino acids. The OppA is a new protein having immunogenicity and can provide effective immunoprotection for Hps infection in mice. The invention also comprises preparation of an Escherichia coli recombinant bacterium BL21 / Hps-oppA expressing the immunogenicity protein gene oppA. The recombinant Hps OppA protein expressed by the invention has favorable safety and protection efficacy, and the immunoprotection effect is up to 60%.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Veterinary compound enrofloxacin injection and preparation method thereof
InactiveCN101658526APromote absorptionEfficient drug deliveryAntibacterial agentsAntipyreticFLUNIXIN MEGLUMINETrimethoprim
The invention relates to a veterinary compound enrofloxacin injection and a preparation method thereof. The veterinary compound enrofloxacin injection mainly comprises enrofloxacin, sulfamethoxazole,trimethoprim, flunixin meglumine, tylosin, organic solvent and water for injection. The veterinary compound enrofloxacin injection is used as a veterinary special compound preparation, used for treating various infectious diseases of a respiratory system, a digestive system and a skin soft tissue caused by livestock and poultry bacterial and mycoplasma infection, particularly has special effects on secondary hemophilus disease of pigs and poultries caused by hemophilus and has the advantages of convenient use, short course, low drug resistance and the like.
Owner:陈建波
Gene chip for identification of six swine disease pathogens and detection method thereof
ActiveCN105063237AStrong and stable hybridization signalStrong specificityMicrobiological testing/measurementMicroorganism based processesSequence analysisDisease
The invention discloses a gene chip for identification of six swine disease pathogens and a detection method thereof. The gene chip is used for detection of porcine actinobacillus pleuropneumoniae, haemophilus parasuis, mycoplasma hyopneumoniae, porcine circovirus type 2, porcine reproductive and respiratory syndrome virus and classical swine fever virus. A PCR primer is designed by means of standard strain genome sequence analysis, cloning and sequencing analysis are carried out on a target gene, and specific probes are designed to perform identification detection on porcine actinobacillus pleuropneumoniae, haemophilus parasuis, mycoplasma hyopneumoniae, porcine circovirus type 2, porcine reproductive and respiratory syndrome virus and classical swine fever virus 6 pathogens at the same time. The invention aims to establish the microarray chip with the advantages of high sensitivity, strong specificity, time saving and labor saving, and easy observation of results to identify and detect the important pathogens of porcine respiratory disease complex.
Owner:重庆海关技术中心
Immunogenic composition
ActiveUS9561268B2Effective protectionBacterial antigen ingredientsMultivalent vaccineStreptococcus pneumoniae conjugatedImmunogenicity
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Antimultiorganism Glycoconjugate vaccine
InactiveUS20050158346A1Enhanced interactionPromotes an increase in inductionBacterial antigen ingredientsSnake antigen ingredientsAntiendomysial antibodiesGlycoconjugate
The present invention relates, e.g., to a glycoconjugate composition comprising one or more polysaccharide types from a cell wall polysaccharide preparation from B. pumilus Sh 18, or variants thereof. Also disclosed are antibodies generated against the glycoconjugates, and methods of using the glycoconjugates and antibodies. An antimultiorganism vaccine which reacts against at least Haemophilus influenzae type a, Haemophilus influenzae type b, Staphylococcus aureus, and Staphylococcus epidermidis, is disclosed.
Owner:UNITED STATES OF AMERICA
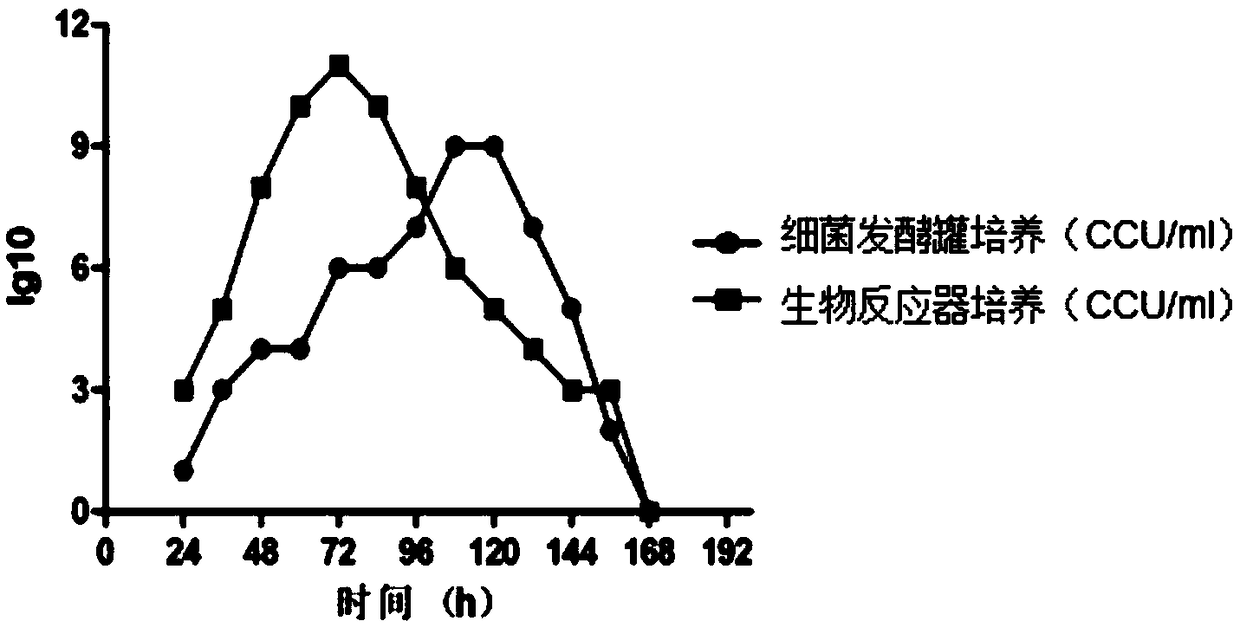
Production method of haemophilus parasuis/mycoplasma hyopneumoniae bivalent inactivated vaccine
ActiveCN109010814AEasy to controlReduce controlAntibacterial agentsBacterial antigen ingredientsSerum igeHaemophilus
The invention belongs to the technical field of vaccine production, and particularly discloses a production method of a haemophilus parasuis / mycoplasma hyopneumoniae bivalent inactivated vaccine. Themethod includes: performing fermenting culture of mycoplasma hyopneumoniae in a bioreactor; performing fermenting culture of serum IV type haemophilus parasuis and serum V type haemophilus parasuis ina fermentation tank; inactivating, concentrating and purifying the microbial liquids after the fermenting cultivation, mixing the microbial liquids according to certain ratio, and adding an immuno-enhancer and a vaccine adjuvant to obtain the haemophilus parasuis / mycoplasma hyopneumoniae bivalent inactivated vaccine. The vaccine composition has excellent specificity and good immunity, can achievethe object of prevention from the two diseases by one injection, is more economical and practicability, can avoid repeated inoculation and reduces vaccine cost and manpower cost. The bivalent inactivated vaccine is very suitable for prevention and treatment on breeding farms with mixed infection of the diseases.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
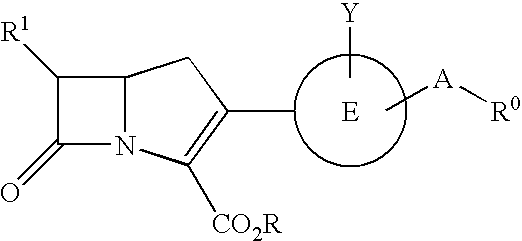
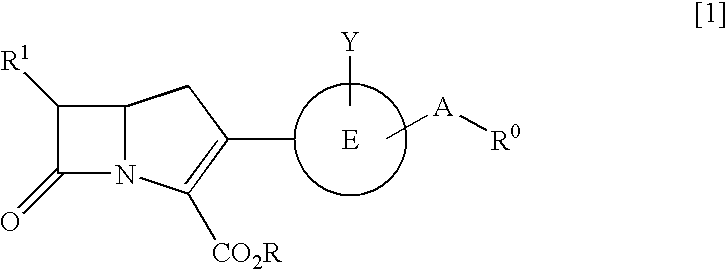
Novel carbapenem compounds
InactiveUS20050020566A1High antibacterial activityPromote oral absorptionAntibacterial agentsBiocideAcquired resistanceAmpicillin
A compound or its pharmaceutically acceptable salt represented by the following formula: The invention is a carbapenem compound which has a potent antibacterial activity over a broad range of Gram negative and Gram positive bacteria, especially penicillin-resistant Streptococcus pneumoniae (PRSP) which has been isolated at an elevated frequency in recent years and thus causes a serious clinical problem, and Haemophilus influenzae which has acquired resistance against the existing β-lactam antibiotics over a wide scope due to penicillin-binding protein (PBP) mutations such as β-lactamase non-producing ampicillin-resistant (BLNAR) Haemophilus influenzae, and has excellent oral absorbability.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Bacterial extract for respiratory disorders and process for its preparation
The present invention relates to an extract from bacterial strains, such as Staphylococcus, Moraxella, Klebsiella, Streptococcus, and Haemophilus. The extract is useful as a treatment for indications such as respiratory disorders, compositions comprising the extract, and processes of making the extract from media that do not pose a risk of prion diseases.
Owner:OM PHARMA
Mucosal combination vaccines for bacterial meningitis
InactiveUS20060147466A1Improving immunogenicityLarge capacityAntibacterial agentsBacterial antigen ingredientsStreptococcus pneumoniaeHaemophilus
A composition for mucosal delivery, comprising two or more of the following: (a) an antigen which induces an immune response against Haemophilus influenzae; (b) an antigen which induces an immune response against Neisseria meningitidis; and (c) an antigen which induces an immune response against Streptococcus pneumoniae. The combination allows a single dose for immunising against three separate causes of a common disease, namely bacterial meningitis.
Owner:CHIRON CORP
Haemophilus parasuis, streptococcus suis and actinobacillus pleuropneumoniae triple inactivated vaccine and application thereof
PendingCN110075289AStrong pathogenicityImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsDiseaseAntigen
The invention relates to the technical field of animal biological products and specifically relates to a haemophilus parasuis, streptococcus suis and actinobacillus pleuropneumoniae triple inactivatedvaccine. The inactivated vaccine includes an inactivated haemophilus parasuis HNHPS1 serotype antigen, an inactivated haemophilus parasuis strain HN1570 serotype antigen, an inactivated streptococcussuis strain HNSS1 serotype antigen and an inactivated actinobacillus pleuropneumoniae strain HNAPP1 antigen; the four strains all have excellent immunogenicity; when the four strain inactivated antigens are used for preparing the vaccine, the infection of clinic haemophilus parasuis, streptococcus suis and actinobacillus pleuropneumoniae can be effectively prevented; the vaccine is characterizedby simple preparation process, high safety, excellent immune effect and long immunity period and meanwhile is capable of achieving the purposes of preventing various diseases with one injection, reducing cost and reducing stress.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE HENAN ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com