Haemophilus parasuis trivalent inactivated vaccine as well as production method and application thereof
A technology of Haemophilus suis and inactivated vaccines, which is applied in the field of Haemophilus parasuis trivalent inactivated vaccines, can solve the problems of low cross-protection rate and achieve high immune efficacy, long immune period and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The screening and identification of embodiment 1 Haemophilus parasuis
[0034] 1. Materials and methods
[0035] 1.1 Source of disease material
[0036] From October 2008 to March 2010, a total of 137 pigs with suspected clinical symptoms of Haemophilus parasuis were collected from Henan, Shandong, Hubei, Anhui, Jiangsu, Hebei and other provinces. Affected pigs often showed fever, dyspnea, joint swelling, lameness, cyanosis of skin and mucous membranes, difficulty in standing or even paralysis, stiff pigs or death. At the same time as the lungs were collected, heart blood, lymph nodes, spleen, brain, joint fluid and other diseased tissues were collected from some pigs for the isolation and identification of bacteria.
[0037] 1.2 Medium
[0038] The preparation method of Tryptic Soy Agar (Tryptic Soy Agar, TSA) medium is as follows: Take 40g of Tryptic Soy Agar, add deionized water, dilute to 1000mL, shake well to dissolve, sterilize by high pressure steam at 121℃ fo...
Embodiment 2
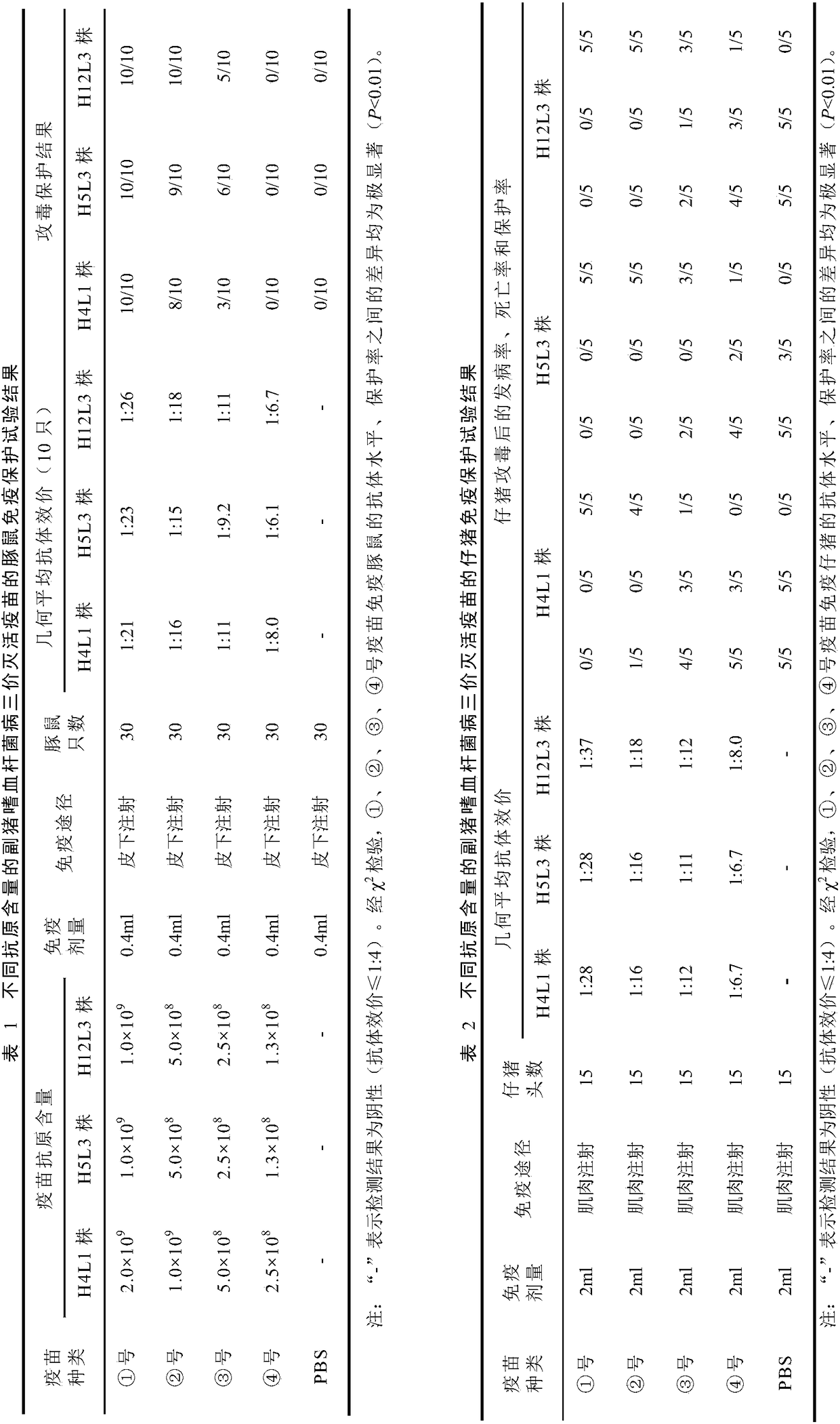
[0073] The production method of embodiment 2 Haemophilus parasuis 4, 5 and 12 type trivalent inactivated vaccines
[0074] 1. Seed Preparation for Production
[0075] (1) First-class seed propagation and identification
[0076] The production strains of Haemophilus parasuis type 4 H4L1 strain, type 5 H5L3 strain and type 12 H12L3 strain were respectively inoculated on TSA plates containing newborn bovine serum (final concentration 10%) and NAD (final concentration 100 μg / mL), Cultivate at 37°C for 24-48 hours, select more than 5 typical colonies for each strain, and inoculate several branches of TSA slant containing newborn bovine serum (final concentration 10%) and NAD (final concentration 100 μg / mL), and culture at 37°C for 24-48 hours. Hours, after passing the pure inspection, it will be regarded as the first-class seed. Stored at 2-8°C, it should not exceed 7 days, and the subculture should not exceed 5 generations.
[0077] (2) Secondary seed propagation
[0078] The ...
Embodiment 3
[0098] Example 3 Haemophilus parasuis 4, 5 and 12 type trivalent inactivated vaccine finished product inspection
[0099] (1) traits
[0100] This product is a gray-white suspension, with a small amount of sediment at the bottom after standing for a long time, and it becomes a homogeneous suspension after shaking.
[0101] (2) Loading inspection
[0102] Tested according to the appendix of the current "Chinese Veterinary Pharmacopoeia", it should meet the requirements.
[0103] (3) Sterility test
[0104] Tested according to the appendix of the current "Chinese Veterinary Pharmacopoeia", it should grow aseptically.
[0105] (4) Safety inspection
[0106] Use 5 healthy susceptible piglets (see Note 4) aged 3 to 4 weeks, inject 4 mL of vaccine into each neck muscle, and observe for 14 days. There should be no local or systemic adverse reactions caused by the vaccine.
[0107] (5) Efficacy test Choose one of the following methods.
[0108] ① Guinea pig immune antibody assay...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com