Production method of haemophilus parasuis/mycoplasma hyopneumoniae bivalent inactivated vaccine
A technology of Mycoplasma hyopneumoniae and double inactivated vaccine, which is applied in the directions of microorganism-based methods, biochemical equipment and methods, vaccines, etc., can solve the problems of increasing labor and production costs, antigen interference or influence, and increasing pollution risks, etc. Achieve the effect of reducing labor costs, reducing electrical use costs, and reducing the risk of microbial contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] 1. Vaccine preparation
[0059] 1. Preparation of seed bacteria
[0060] (1) First-class seed propagation and identification
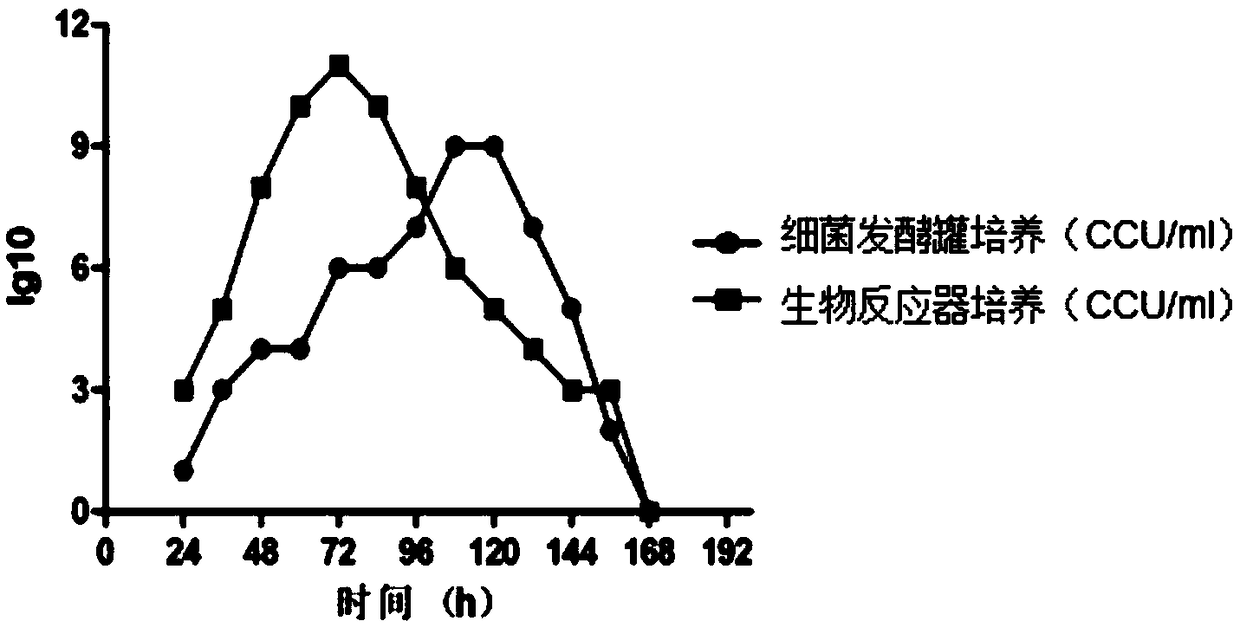
[0061] The freeze-dried bacteria of Haemophilus parasuis serotype 4 strain MD0322 and the freeze-dried strain of Haemophilus parasuis serotype 5 SH0165 were streaked and inoculated on TSA agar plates, and cultured at a constant temperature of 37 °C for 20-24 hours. Typical bacterial colonies or bacterial lawns are produced, and after passing the pure inspection, they are used as first-class seeds and stored at 2-8°C for no more than 7 days; freeze-dried strains of Mycoplasma hyopneumoniae XJ03 strain are inoculated with 100ml / bottle of Mycoplasma liquid culture medium medium, cultured at 37°C for 3 to 5 days, when the color of the medium turned yellow, slightly turbid, and the pH value dropped to 6.5-6.8, the bacterial liquid was harvested, and it was regarded as the first-class seed after passing the pure inspection. The specific parameters ar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com