Haemophilus parasuis LC strain and application thereof
A technology of Haemophilus suis and bacteria content, applied in the direction of bacteria, antibacterial drugs, medical preparations containing active ingredients, etc., to achieve the effects of reducing morbidity and mortality, reducing economic losses, preventing epidemics and spreading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Haemophilus parasuis ( Haemophilus parasuis ) LC strain, the preservation number of which is CGMCC No. 5257 in the General Microbiology Center of China Committee for Culture Collection of Microorganisms.
[0028] production medium
[0029] Preparation of TSA medium: Weigh 4 g of TSA powder and add it to 100 mL of distilled water, mix thoroughly, sterilize at 121°C for 15 min, cool to 50°C-60°C, add final concentration of 0.001% NAD and 5% newborn bovine serum , mix well and pour into a flat plate for later use.
[0030] Preparation of TSB medium: Weigh 3 g of TSB powder into 100 mL of distilled water, and sterilize at 121 °C for 15 min. It is prepared by adding final concentration of 0.001% NAD and 5% newborn bovine serum before use, and mixing evenly.
[0031] 1.1 Isolation and purification method of HPS strain
[0032] Sick pigs were selected from a large pig farm suspected of Haemophilus parasuis disease in Liaocheng, Shandong Province, and the pericardial effus...
Embodiment 2
[0056] Haemophilus parasuis ( Haemophilus parasuis ) Application of LC strain in the prepared Haemophilus parasuis inactivated vaccine.
[0057] Get the Haemophilus parasuis (Hemophilus parasuis) whose strain preservation number is CGMCC No. 5257 Haemophilus parasuis ) LC strain, inoculate the culture medium after activation, collect the bacterial liquid after culture, inactivate with formaldehyde solution, and mix with aluminum hydroxide gel adjuvant to prepare inactivated aluminum gel vaccine.
[0058] The preferred operation steps are as follows:
[0059] 2.1 Strain selection
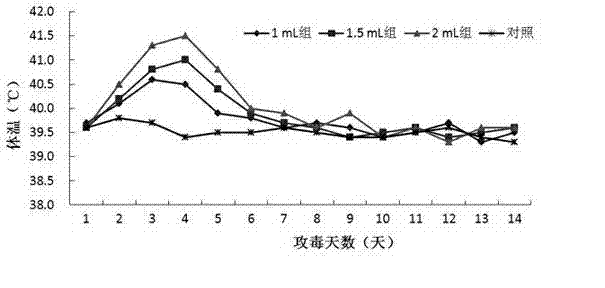
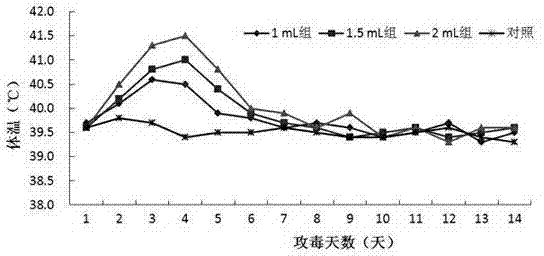
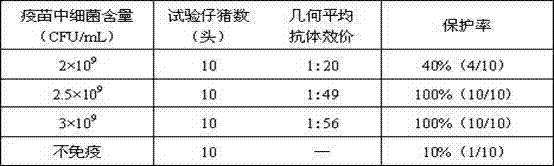
[0060] The strain used for making seedlings is HPS LC strain, and its preservation number is CGMCC No.5257. Its colony morphology, bacterial characteristics, biochemical characteristics, and culture characteristics are all stable, and it has strong pathogenicity to pigs. 1.5 mL of bacterial liquid ( 3.0×10 9 CFU / mL) intraperitoneal injection of piglets can cause 80% of piglets to develop disea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com