Porcine circovirus type 2, mycoplasma hyopneumoniae and haemophilus parasuis triple inactivated vaccine and preparation method thereof
A technology of Mycoplasma hyopneumoniae and Haemophilus suis, which is applied in the direction of virus antigen components, virus/phage, biochemical equipment and methods, etc., can solve problems such as difficulty in guaranteeing 100% activity, lack of processing and modification, and improve pig production Good performance, good mental state, good protein activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of embodiment 1 porcine circovirus type 2, mycoplasma hyopneumoniae, Haemophilus parasuis triple inactivated vaccine, such as Figure 5 Shown:
[0048] 1. Preparation of porcine circovirus type 2 semi-finished antigen
[0049] 1) The preferred eukaryotic expression plasmid containing circovirus type 2 cap protein was electrotransformed into Pichia pastoris cells (X-33, purchased from Invitrogen, USA). Positive clone seeds were screened out, inoculated in 250ml of BMGY liquid medium, 30°C, 250r / min shaking culture for 16h-24h; after that, 250ml of proliferated seed liquid was added to sterilized FBSM inorganic salt medium, and fermented in a fermenter Expanded cultivation in. When the OD600 of the bacterial liquid was 1.0, 1% methanol was added to induce it for 80h-96h, and the bacterial liquid was harvested.
[0050] 2) The yeast harvested liquid was centrifuged to remove the supernatant, and the precipitate was re-selected with 0.01M PBS solution and c...
Embodiment 2
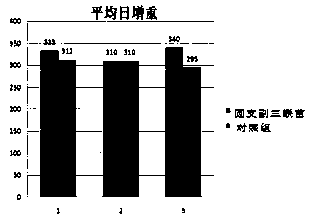
[0068] Example 2 Safety Test of Porcine Circovirus Type 2, Mycoplasma Hyopneumoniae, and Haemophilus Parasuis Triple Inactivated Vaccine
[0069] 1. Guinea pig security inspection: Divide 180-220g guinea pigs into two groups, 8 in each group. In the test group, each guinea pig was intraperitoneally injected with 5ml of porcine triple vaccine; in the control group, each guinea pig was intraperitoneally injected with 5ml of normal saline, and observed continuously for 7 days. All the guinea pigs in the two groups survived without local or systemic adverse reactions. The results are shown in Table 1. .
[0070] Table 1 pig triple vaccine of the present invention is in the safety inspection result of guinea pig
[0071]
[0072]
[0073] 2. Piglet security check: screen three litters of healthy piglets for 14-21 days, select 6 piglets in each litter, inject 2ml of the pig triple vaccine of the present invention into the neck muscle, observe continuously for 2 hours, and no ...
Embodiment 3
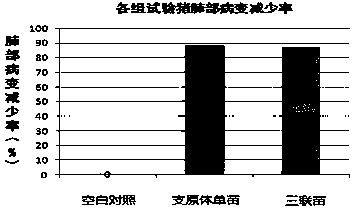
[0074] Example 3 The protective effect of pig triple vaccine on circovirus type 2 and Haemophilus parasuis in mice
[0075] 1. The protective effect of triple vaccine against circovirus type 2 challenge
[0076] 30 18-22g BALB / C mice were fed for one week and then divided into 3 groups, namely triple vaccine group, single circular vaccine group and blank control group. Each mouse was intraperitoneally injected with 0.5ml vaccine, and the control group was injected with the same dose of PBS solution. After 14 days, booster immunization was carried out according to the same method, with a dose of 0.5ml / mouse.
[0077] Twenty-one days after BALB / c mice were immunized, together with 10 control mice, the DBN-SX07 strain was used to test the virus (≥10 7.0 TCID 50 / ml) challenge, each mouse was intraperitoneally injected with 0.5ml, and 21 days after the challenge, the spleen was culled and taken for virus isolation. If the virus was isolated, it was judged to be positive for viru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com