Hemophilus parasuis disease, swine streptococcosis bivalent inactivated vaccine and preparation method thereof
A technology of haemophilus suis disease and double inactivated vaccine, which is applied in the direction of antibacterial drugs, pharmaceutical formulas, bacterial antigen components, etc. Questions about vaccine antigens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: Preparation of Haemophilus parasuis, Streptococcus suis dual inactivated vaccine (JS strain+ZJ strain+SC strain)
[0059] 1. Preparation of strains for production
[0060] Haemophilus parasuis serotype 4 JS strain, type 5 ZJ strain and Streptococcus suis type 2 SC strain were propagated by primary seed and secondary seed propagation to obtain Haemophilus parasuis serotype 4 JS strain and type 5 ZJ strain strains and Streptococcus suis type 2 SC strains for production.
[0061] (1) Primary seed propagation
[0062] Primary seed propagation was carried out on Haemophilus parasuis serotype 4 JS strain, type 5 ZJ strain and Streptococcus suis type 2 SC strain. Streak and inoculate the freeze-dried strains of Haemophilus parasuis serotype 4 JS strain and type 5 ZJ strain on the TSA / NAD plate, culture them at 37°C for 18 hours, select colonies that meet the requirements, and inoculate them on TSB / NAD liquid medium, cultivated at 37°C for 12 hours, as the pri...
Embodiment 2
[0097] Embodiment 2: safety research
[0098] (1) Safety test of the vaccine on Balb / C mice
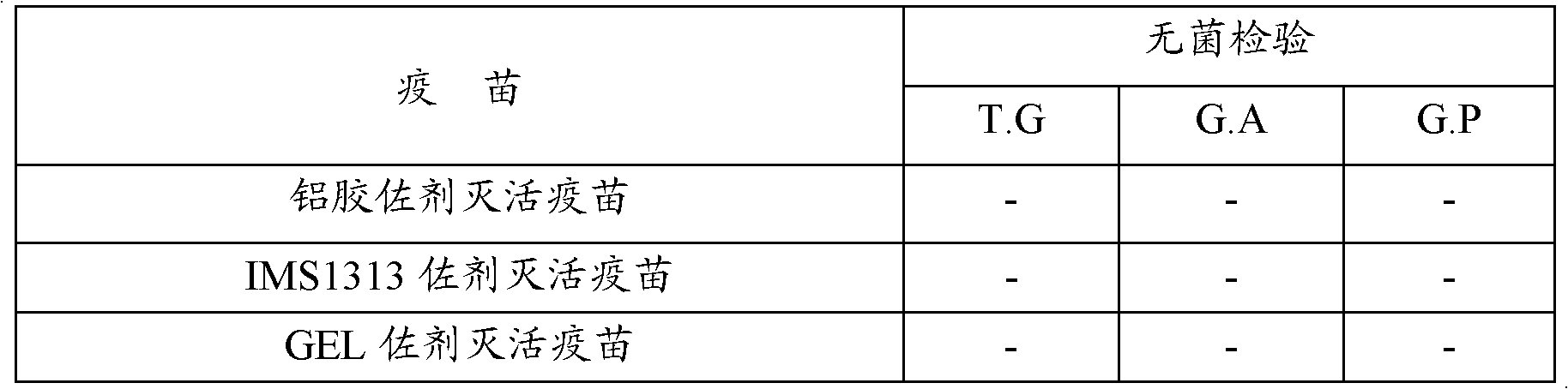
[0099] The vaccine prepared in Example 1 was used to inoculate 10 Balb / C mice of 18 to 22 g each, each subcutaneously injected with 0.4 ml, and observed for 14 days. There was no local reaction and all were healthy and alive, as shown in Table 2.
[0100] Table 2 vaccine is to Balb / C mouse safety test result
[0101]
[0102] (2) Safety test of vaccine on piglets
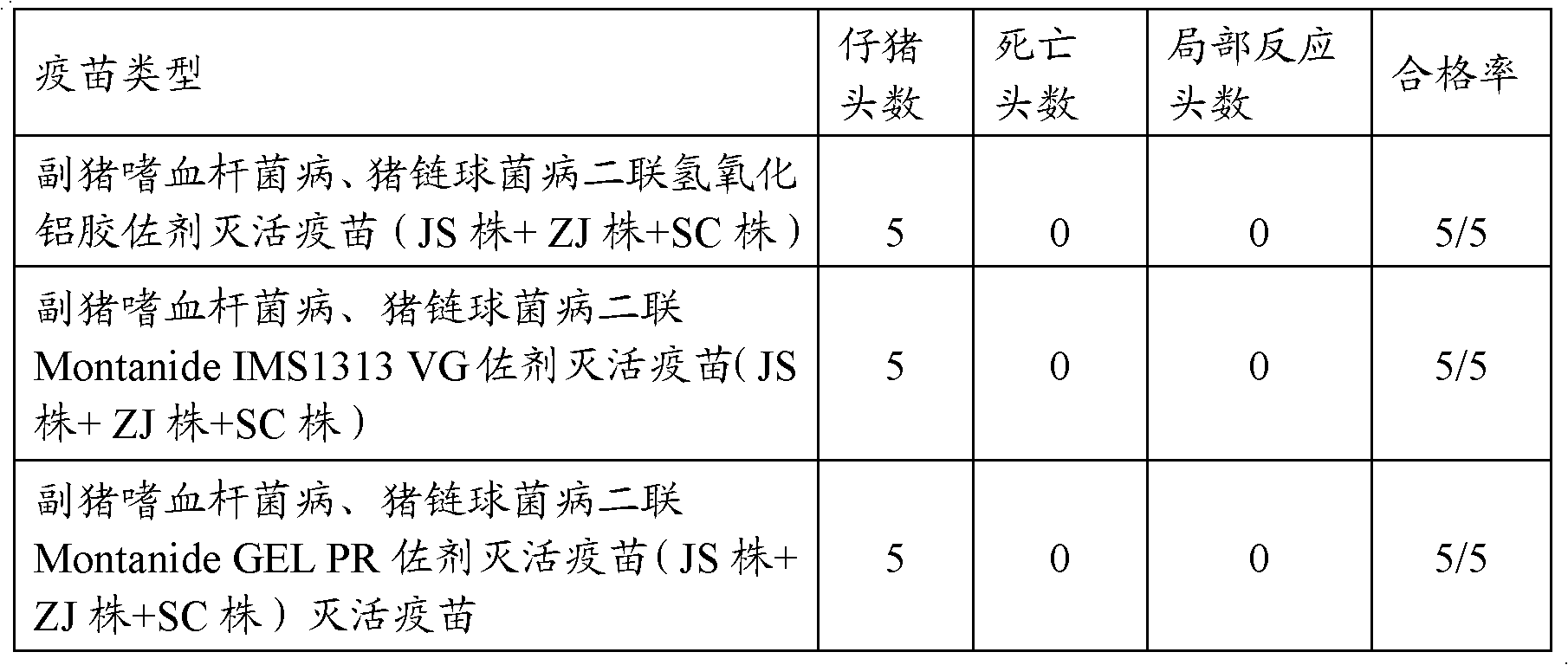
[0103] The vaccine prepared in Example 1 was injected intramuscularly into 5 30-day-old healthy susceptible pigs, each with 4 mL, and there was no local reaction within 14 days and all were healthy and alive. The test results are shown in Table 3.
[0104] Table 3 vaccine is to the safety test result of piglet
[0105]
[0106]
[0107] It can be seen from the safety test results that Haemophilus parasuis, Streptococcus suis double aluminum hydroxide gel adjuvant inactivated vaccine (JS strain + ZJ strain + SC ...
Embodiment 3
[0108] Embodiment 3: Efficacy test (tested in our company's experimental animal room)
[0109] (1) Efficacy test in Balb / C mice
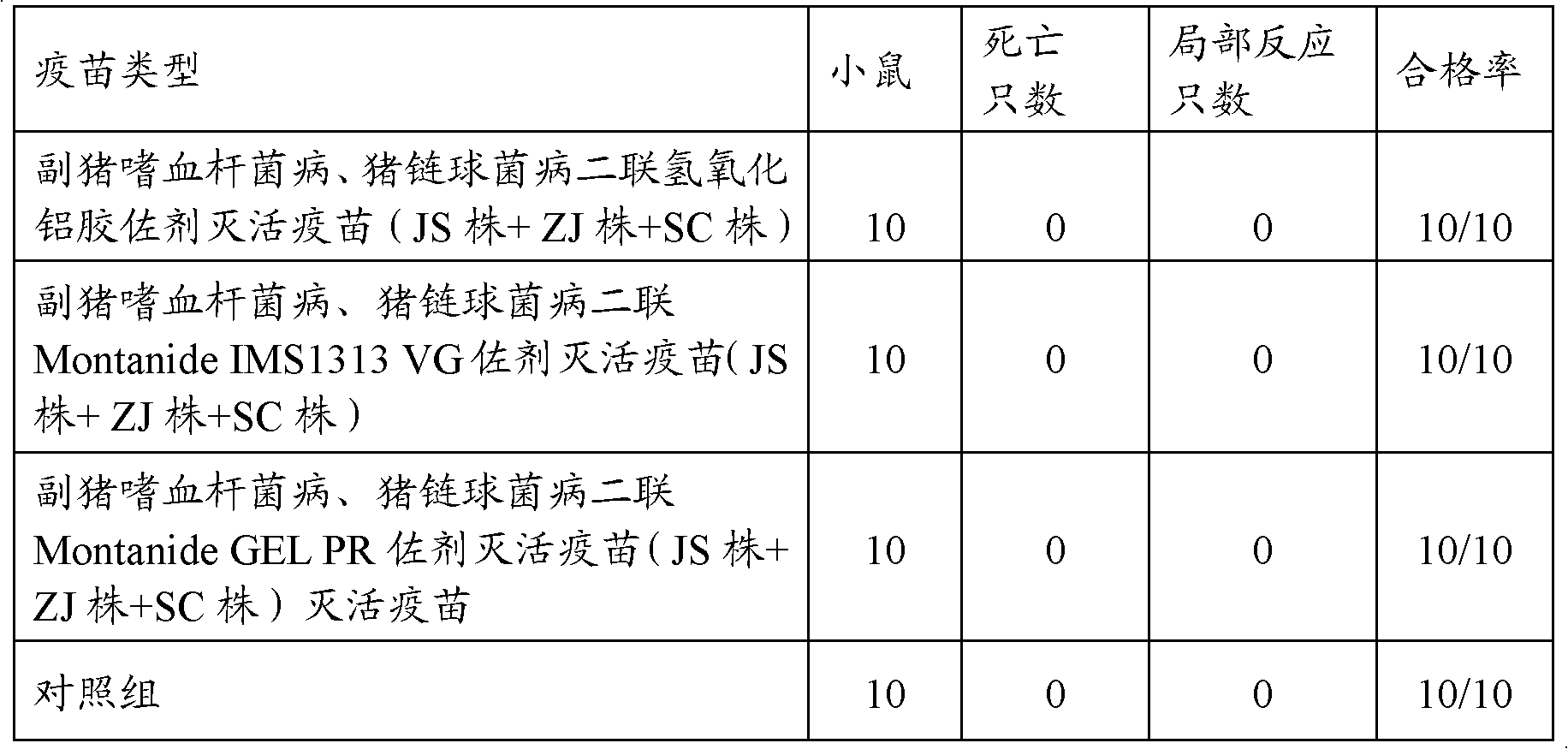
[0110] Balb / C mice weighing 18-22 g were subcutaneously inoculated with 0.1 mL of double inactivated vaccine, and divided into 3 groups 21-28 days after immunization, together with 10 mice in each group of the control group, and treated with Haemophilus parasuis serum type 4 JS Intraperitoneally inject 1ml of the strain (the bacteria content is 4.0×10 9 CFU), type 5 ZJ strains were intraperitoneally injected with 1ml (the bacterial content was 2.5×10 9 CFU); and subcutaneous injection of 1ml of Streptococcus suis type 2 SC strain (the bacterial content is 1.0×10 2 CFU), another 10 were set as blank control, observed for 14 days, and the test results are shown in Table 4.
[0111] Table 4Balb / C mouse potency test result
[0112]
[0113] (2) Piglet efficacy test
[0114] Take the prepared vaccine, use 15 healthy susceptible pigs aged 28-35 da...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com