Swine streptococcicosis-Glasser's disease bivalent subunit vaccine and preparation method thereof
A technology for Haemophilus suis and subunit vaccines, which can be applied to vaccines, multivalent vaccines, and veterinary vaccines, and can solve problems such as poor cross-protection effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The screening of embodiment one Streptococcus suis antigenic protein
[0052] 1 Genome extraction of Streptococcus suis
[0053] Streptococcus suis 05ZYH33 glycerol bacteria preserved in the laboratory were lined in THA medium (containing 10% horse serum), then placed in a constant temperature incubator at 37°C overnight, and a single colony was picked and transferred to THB medium (containing 10% horse serum). % horse serum) in an incubator for static culture, and then expand the culture to 10mL THB medium (containing 10% horse serum), and use the bacterial genome extraction kit to extract the bacterial genome according to the instructions. The extracted genome was stored in a -20 refrigerator for future use.
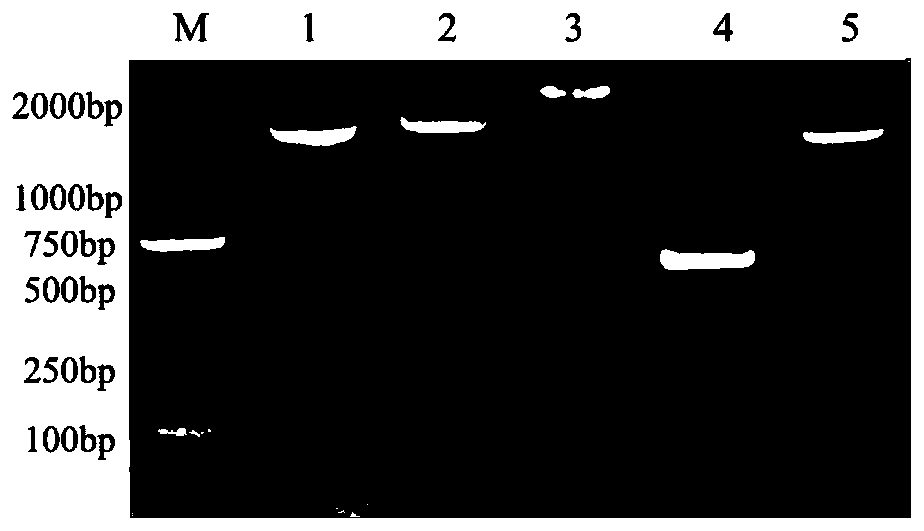
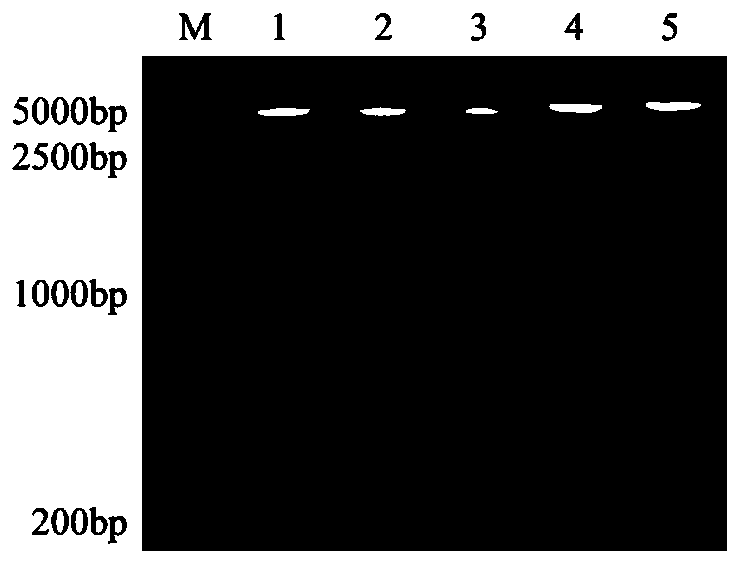
[0054] 2 Construction and verification of recombinant plasmids
[0055]Primers were designed according to the gene sequence of strain 05ZYH33 in GenBank (accession number CP000407.1) (Table 2-1). Using the genome of the standard strain 05ZYH33 as a template, ...
Embodiment 2
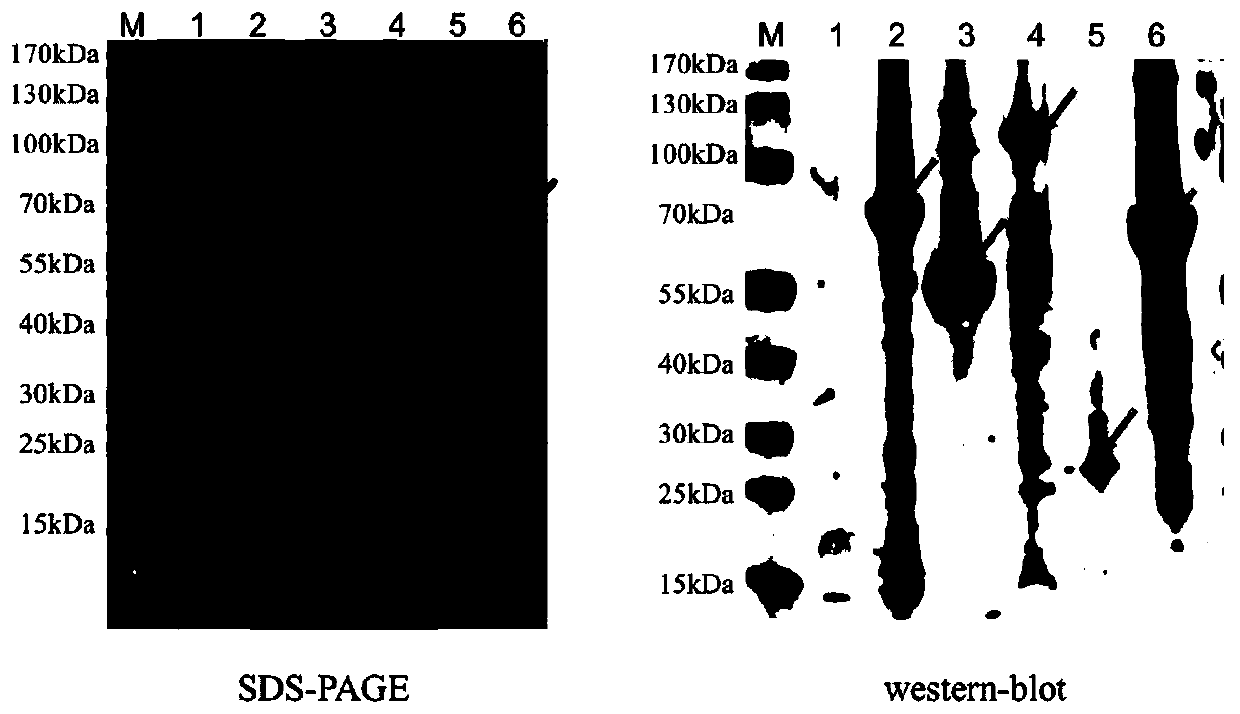
[0090] Example 2 Construction and Verification of Surface Display Carriers
[0091] According to the aforementioned experimental results, in this example, two exogenous proteins of Streptococcus suis antigens MRP and SLY were displayed on the surface of E.coli BL21 (DE3) cells, and AfuA, CdtB, and OppA of Haemophilus parasuis were used. , OppA2 displayed on the surface of Escherichia coli and evaluated its immunogenicity through animal experiments.
[0092] 1 Related primer design
[0093] Linker is a 3-time repeated sequence of 5 amino acids GGGGS that separates two protein sequences, and the linkers at both ends need to be complementary to the base sequences at both ends of the protein. The linearized vector used for cloning should use the pMD-28a-INP-CAP-His plasmid as a template for reverse amplification, and the sequences at both ends should also be complementary to the terminal base sequences of the proteins connected on both sides. Based on the HPS gene sequence (NC_011...
Embodiment 3
[0117] Embodiment 3 animal test
[0118] 1 Vaccine preparation
[0119] 1.1 Surface-displayed dual subunit vaccines
[0120] (1) Shaking bacteria: Take the constructed glycerol bacteria displaying the recombinant strain on the surface, draw lines on LB (containing Amp 100 μg / mL) plates, and place them in a 37°C incubator for 8 hours. On the next day, single clones were picked and inoculated in LB (containing Amp 100 μg / mL) liquid medium, cultured at 37°C with shaking at 180 r / min, and then transferred to 500 mL of the corresponding medium at a ratio of 1:100 , shake at 180r / min in a constant temperature shaker at 37°C.
[0121](2) Bacteria collection and inactivation: collect the bacteria under sterile conditions and wash them with PBS for 3 times, then add an appropriate amount of PBS to resuspend, add formaldehyde with a working concentration of 1.5‰ to inactivate the bacteria, and check whether the bacteria are inactivated after 48 hours completely.
[0122] (3) Quantif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com