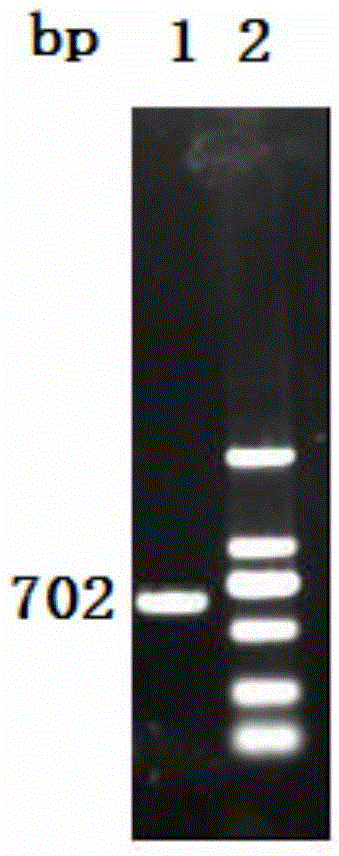
Vaccine composition containing porcine circovirus type 2 antigen and haemophilus parasuis antigen and preparation method and application thereof
A technology of Haemophilus suis and vaccine composition, applied in the field of vaccine composition for pigs, can solve the problems of complicated epidemic prevention tasks, increased animal stress, immune failure and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] The preparation and inspection of the vaccine composition of embodiment 1 porcine circovirus-related diseases and Haemophilus parasuis disease
[0090] 1.1 Preparation of bacteria (poison) species for production
[0091] The preparation of porcine circovirus type 2 SH: the virus seed PCV2SH strain was diluted 1:9 with MEM liquid medium (prepared according to the instruction manual with the MEM dry powder medium purchased from Invitrogen Company of the United States), and then diluted by 5% of the volume of the cell culture medium. % inoculated on PK15 (ATCC, preservation number is CCL-33) monolayer cells, adsorbed at 37°C for 30 minutes, added cell maintenance solution (add 4% calf serum and 2mmol / L D-glucosamine hydrochloride in MEM liquid medium) ), cultured at 37°C for 4 days, frozen and thawed 2 to 3 times, and the virus was harvested, and the virus titer was 10 6.5 TCID 50 / ml.
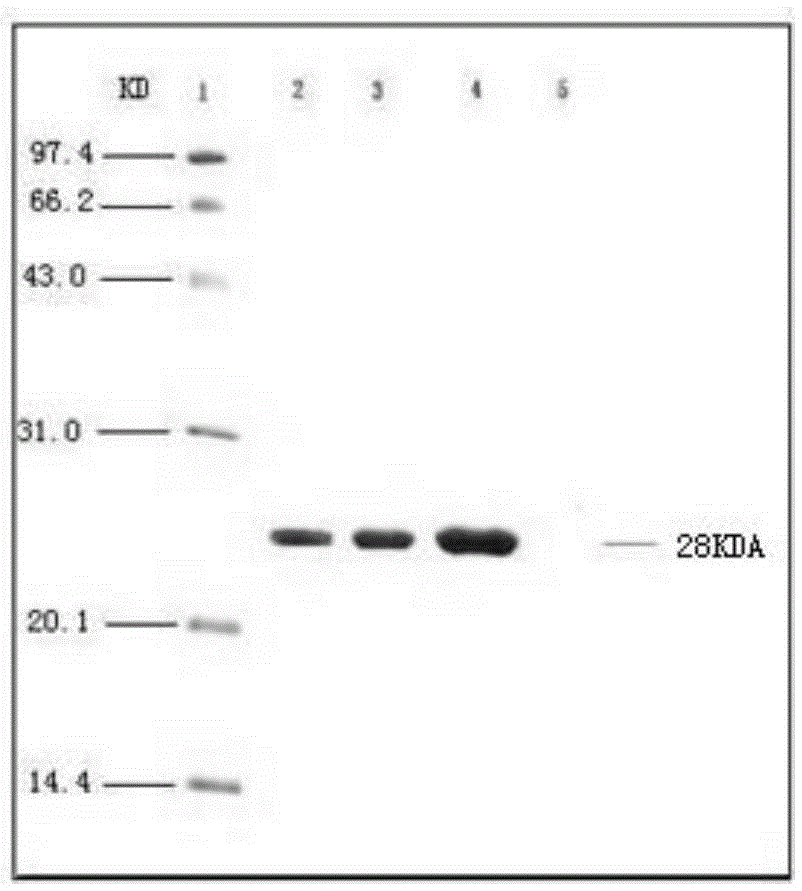
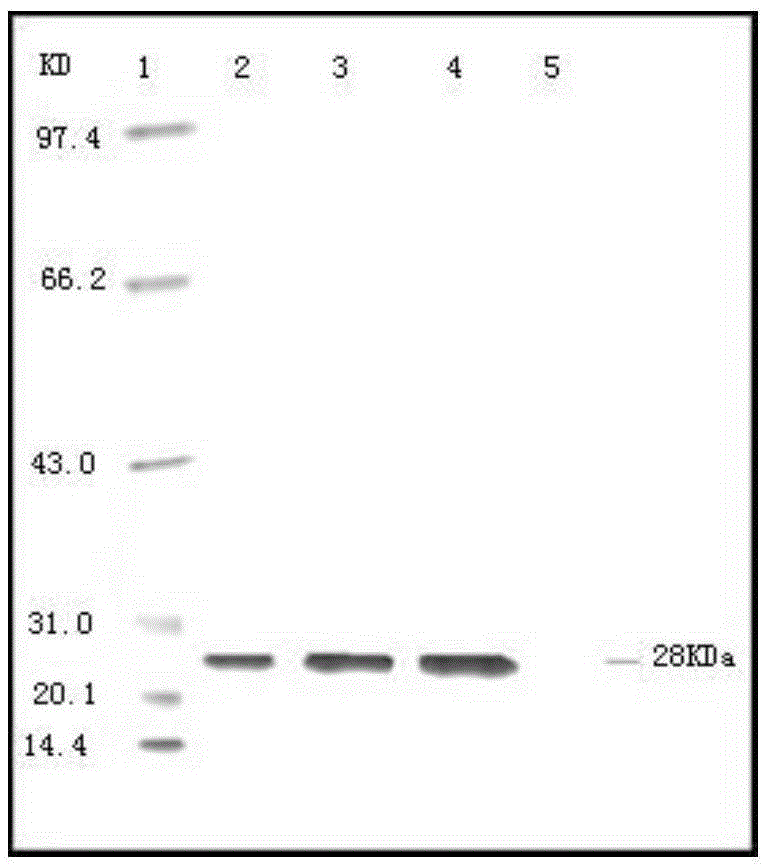
[0092]Preparation of Haemophilus parasuis strains: Streak inoculation of Haemophilu...
Embodiment 2
[0151] Embodiment 2: the preparation of porcine circovirus type 2 antigen, Haemophilus parasuis bivalent (serum type 4+serum type 5) antigen vaccine composition
[0152] Adopt embodiment 1 to prepare (i.e. K001 batch of antigens, see Table 2) porcine circovirus type 2 inactivated virus liquid and the inactivated bacterial liquid of Haemophilus parasuis (serum type 4 JS strain and serum type ZJ strain), Prepare the vaccine according to the following formula:
[0153] Inactivated PCV2 virus liquid (the virus content before inactivation is 10 6.0 TCID 50 / ml) 45ml
[0154] Inactivated Haemophilus parasuis type 4 bacteria liquid (the content of the bacteria liquid before inactivation is 4.5×10 9.0 cfu / ml) 22.5ml
[0155] Inactivated Haemophilus parasuis type 5 bacteria liquid (the content of the bacteria liquid before inactivation is 4.5×10 9.0 cfu / ml) 22.5ml
[0156] Montanide TM Gel10ml
[0157] Put the inactivated virus liquid and the bacterium liquid (prepared in embod...
Embodiment 3
[0158] Example 3: Comparison of PCV2 inactivated whole virus antigen vaccine against PCV2 challenge immune effect with PCV2 inactivated virus antigen and Haemophilus parasuis inactivated antigen vaccine composition
[0159] Antigens prepared in Example 1 (i.e. K001 batch of antigens, see Table 2) were used to prepare porcine circovirus type 2, Haemophilus parasuis (serum type 4+serum type 5) vaccine compositions (V1) and (V2), respectively Porcine circovirus vaccine (V3) and (V4), the specific formula is as follows.
[0160] (1) Porcine circovirus type 2, Haemophilus parasuis (serotype 4 + serotype 5) vaccine composition (V1)
[0161]
[0162] (2) Porcine circovirus type 2, Haemophilus parasuis (serotype 4 + serotype 5) vaccine composition (V2) total volume 100ml
[0163] Inactivated PCV2 virus solution (containing 10 6.0 TCID 50 / ml) 22.5ml
[0164] Inactivated Haemophilus parasuis type 4 bacterial liquid (containing 4.5×10 9.0 cfu / ml) 11.25ml
[0165] Inactivated Ha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com