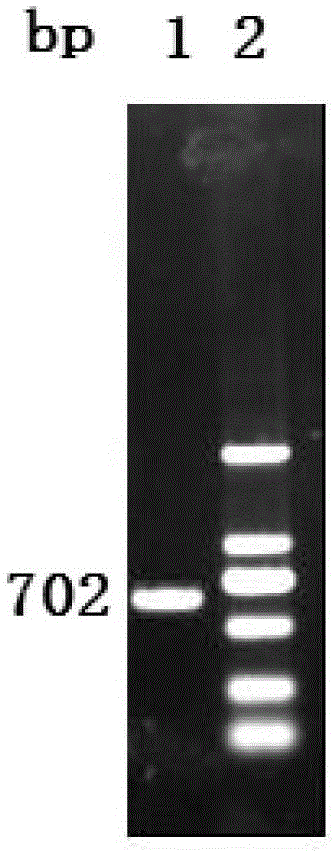
Vaccine composition containing porcine circovirus type 2 antigen and Haemophilus parasuis antigen and its preparation method and application
A technology of Haemophilus suis and porcine circovirus, which is applied in biochemical equipment and methods, medical preparations containing active ingredients, and microorganism-based methods, etc., can solve the problems of immunization failure, insufficient time interval, and increase the cost of epidemic prevention.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
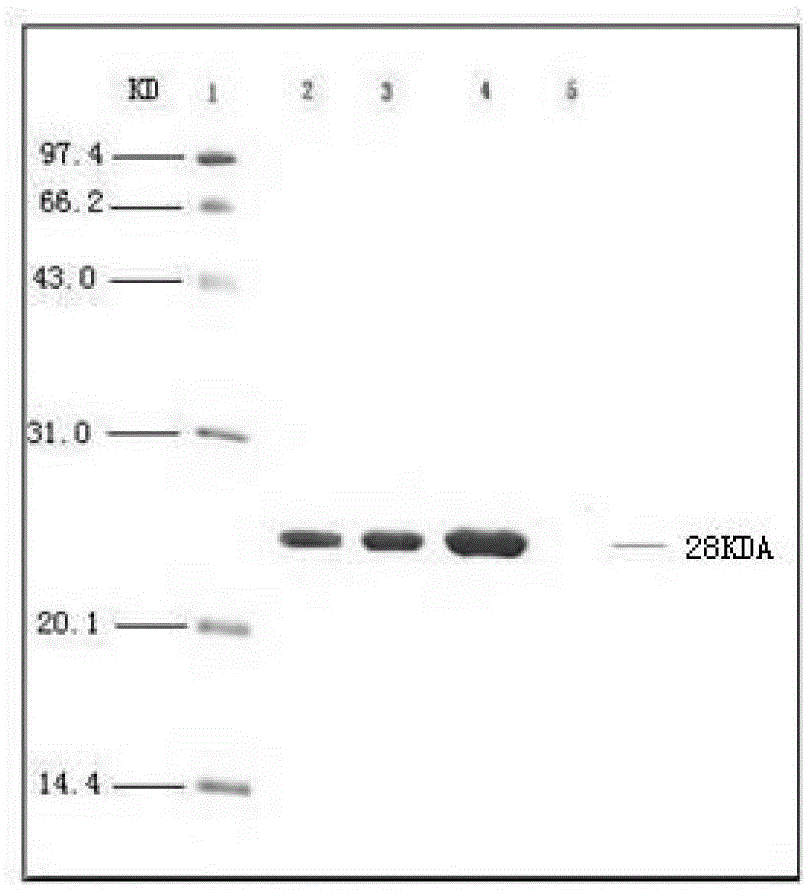
Image
Examples
Embodiment 1
[0088] The preparation and inspection of the vaccine composition of embodiment 1 porcine circovirus-related diseases and Haemophilus parasuis disease
[0089] 1.1 Preparation of bacteria (virus) species for production
[0090] Preparation of porcine circovirus type 2 SH: the virus seed PCV2SH strain was diluted 1:9 with MEM liquid medium (prepared with MEM dry powder medium purchased from Invitrogen Company of the United States according to the instructions), and then divided into 5% of the volume of cell culture medium. % inoculated in PK15 (ATCC, preservation number CCL-33) monolayer cells, adsorbed at 37°C for 30 minutes, added cell maintenance solution (4% calf serum and 2mmol / L D-glucosamine hydrochloride were added to MEM liquid medium) ), cultivated at 37°C for 4 days, freeze-thawed 2-3 times, harvested the virus, and the virus titer was 10 6.5 TCID 50 / ml.
[0091]Preparation of Haemophilus parasuis strains: Streak inoculation of Haemophilus parasuis type 4 JS strai...
Embodiment 2
[0149] Example 2: Preparation of porcine circovirus type 2 antigen and Haemophilus parasuis bivalent (serotype 4 + serotype 5) antigen vaccine composition
[0150] Prepared by Example 1 (i.e. K001 batch of antigens, see Table 2) porcine circovirus type 2 inactivated virus liquid and Haemophilus parasuis (serum type 4 JS strain and serum type 5 ZJ strain) inactivated bacterial liquid, Prepare the vaccine according to the following formula:
[0151] Inactivated PCV2 virus liquid (the virus content before inactivation is 10 6.0 TCID 50 / ml) 45ml
[0152] Inactivated Haemophilus parasuis type 4 bacteria liquid (the content of the bacteria liquid before inactivation is 4.5×10 9.0 cfu / ml) 22.5ml
[0153] Inactivated Haemophilus parasuis type 5 bacteria liquid (the content of the bacteria liquid before inactivation is 4.5×10 9.0 cfu / ml) 22.5ml
[0154] Montanide TM Gel10ml
[0155] Put the inactivated virus liquid and the inactivated bacterial liquid (prepared in Example 1) i...
Embodiment 3
[0156] Example 3: Comparison of PCV2 inactivated whole virus antigen vaccine against PCV2 challenge immune effect with PCV2 inactivated virus antigen and Haemophilus parasuis inactivated antigen vaccine composition
[0157] The antigens prepared in Example 1 (i.e. the K001 batch of antigens, see Table 2) were used to prepare porcine circovirus type 2, Haemophilus parasuis (serotype 4 + serotype 5) vaccine compositions (V1) and (V2), respectively. Porcine circovirus vaccine (V3) and (V4), the specific formula is as follows.
[0158] (1) Porcine circovirus type 2, Haemophilus parasuis (serotype 4 + serotype 5) vaccine composition (V1)
[0159] Inactivated PCV2 virus liquid (containing 10 6.0 TCID 50 / ml) 45ml
[0160] Inactivated Haemophilus parasuis type 4 bacteria liquid (containing 4.5×10 9.0 cfu / ml) 22.5ml
[0161] Inactivated Haemophilus parasuis type 5 bacteria liquid (containing 4.5×10 9.0 cfu / ml) 22.5ml
[0162] Montanide TM Gel10ml
[0163] (2) Porcine circovir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com