Anti-porcine atrophic rhinitis and haemophilus parasuis vaccine composition and its preparation
A vaccine composition, the technology of Haemophilus suis, is applied in the field of animal husbandry biopharmaceuticals, and can solve the problems of inability to protect pigs, asynchronous immunity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
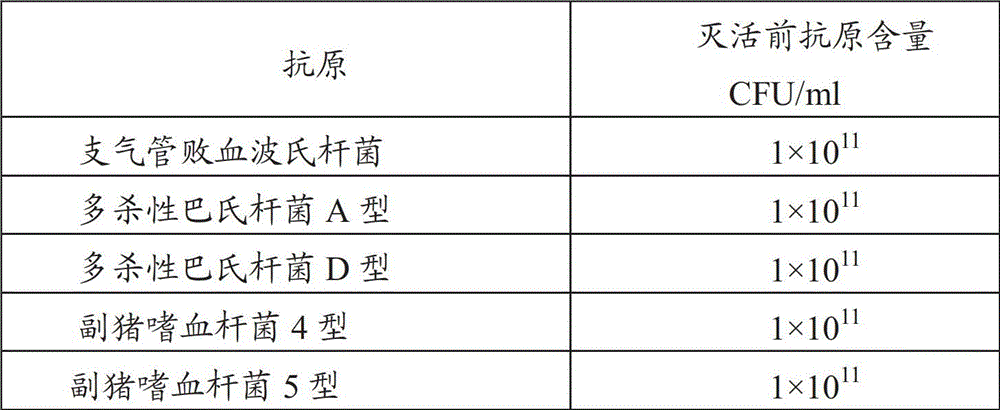
[0061] Example 1: Preparation of porcine bronchiseptica Bordetella antigen, Pasteurella multocida antigen and Haemophilus parasuis antigen
[0062] 1. Source of the strain
[0063] Bordetella bronchiseptica HN8 strain (CCTCC NO: M2011223), Pasteurella multocida type A HN5 strain (CCTCC NO: M2011222) and D type HB4 strain (CCTCC NO: M2011221) used in the manufacture and inspection of this product, Haemophilus parasuis type 4 JS strain (CCTCC M 2011172) and type 5 ZJ strain (CCTCC M 2011173) were both isolated and identified by Pulaike Bioengineering Co., Ltd. Preserved by the Culture Collection Center.
[0064] The strains used in the manufacture and inspection of this product are Bordetella bronchiseptica HN8 strain, Pasteurella multocida type A HN5 strain and D type HB4 strain, Haemophilus parasuis type 4 JS strain and type 5 ZJ strain, All were stored freeze-dried.
[0065] 2. Preparation and inspection of vaccine semi-finished products
[0066] (1) Preparation of seeds ...
Embodiment 2
[0088] Embodiment 2: Preparation of anti-porcine atrophic rhinitis and Haemophilus parasuis disease vaccine composition
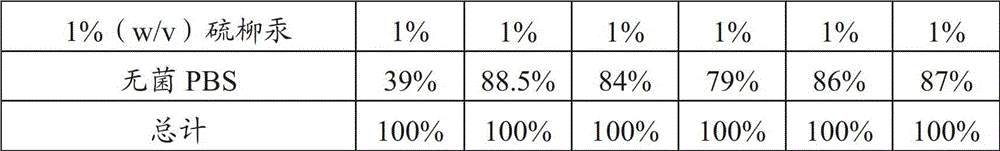
[0089] 1. Preparation of preservatives
[0090] 1% (w / v) thimerosal aqueous solution: 1 g of thimerosal was dissolved in 100 ml of purified water, and autoclaved at 121° C. for 30 minutes for later use.
[0091] 2. Preparation of diluent
[0092] Sterile PBS buffer solution: Dissolve 8g sodium chloride, 0.25g potassium chloride, 3.63g disodium hydrogen phosphate, 0.24g potassium dihydrogen phosphate in 900ml purified water, then dilute to 1L, autoclave at 121°C for 30min spare.
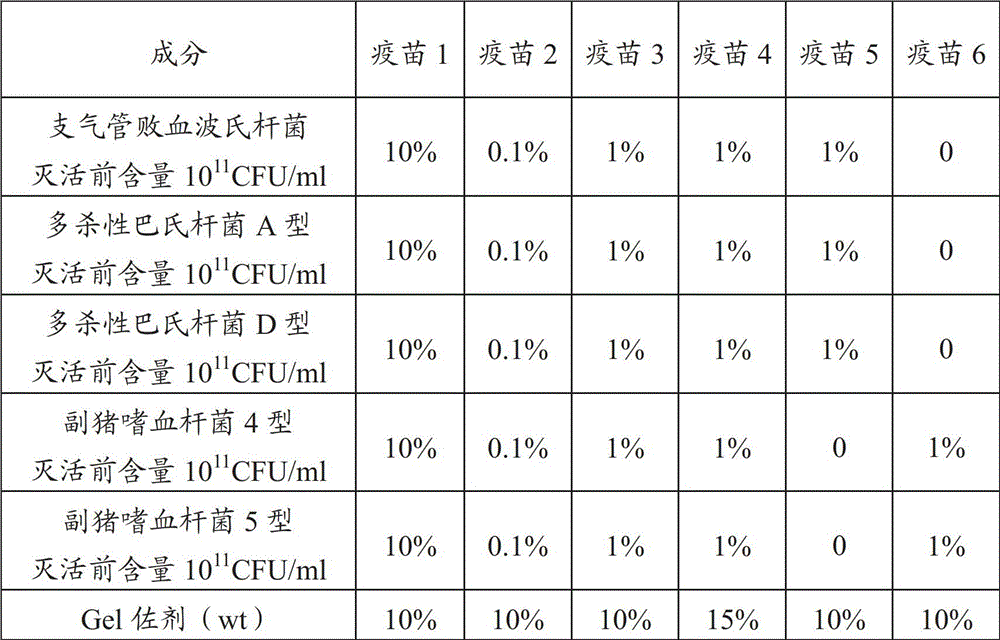
[0093] 3. Vaccine Adjuvant Treatment
[0094] Sterilization of the Gel adjuvant: transfer the Gel adjuvant into a sterilizable container, and autoclave at 121°C for 30 minutes for later use.
[0095] 4. Matching seedlings
[0096] Mix the above ingredients in a certain proportion, that is, through aseptic operation, the concentrated antigen of Bordetella bronchiseptica, the co...
Embodiment 3
[0100] Example 3: Anti-porcine atrophic rhinitis and Haemophilus parasuis vaccine composition efficacy test with different antigen contents
[0101] 1. Test material
[0102] According to embodiment 2 laboratory preparation anti-porcine atrophic rhinitis and Haemophilus parasuis vaccine composition, vaccine 1 (bordetella porcine bronchiseptica antigen content 10 10 CFU / ml, Pasteurella multocida type A antigen content 10 10 CFU / ml, Pasteurella multocida type D antigen content 10 10 CFU / ml, Haemophilus parasuis type 4 antigen content 10 10 CFU / ml, Haemophilus parasuis type 5 antigen content 10 10 CFU / ml) and vaccine 2 (Bordetella porcine bronchiseptica antigen content 10 8 CFU / ml, Pasteurella multocida type A antigen content 10 8 CFU / ml, Pasteurella multocida type D antigen content 10 8 CFU / ml, Haemophilus parasuis type 4 antigen content 10 8 CFU / ml, Haemophilus parasuis type 5 antigen content 10 8 CFU / ml).
[0103] Weaned piglets aged 3 to 4 weeks without antibodies to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com