Serotype 7 haemophilus parasuis natural weak virulent strain and application thereof
A Haemophilus suis, natural technology, used in bacteria, antibacterial drugs, medical preparations containing active ingredients, etc. The effect of high drug production efficiency, simple preparation method and weakened pathogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
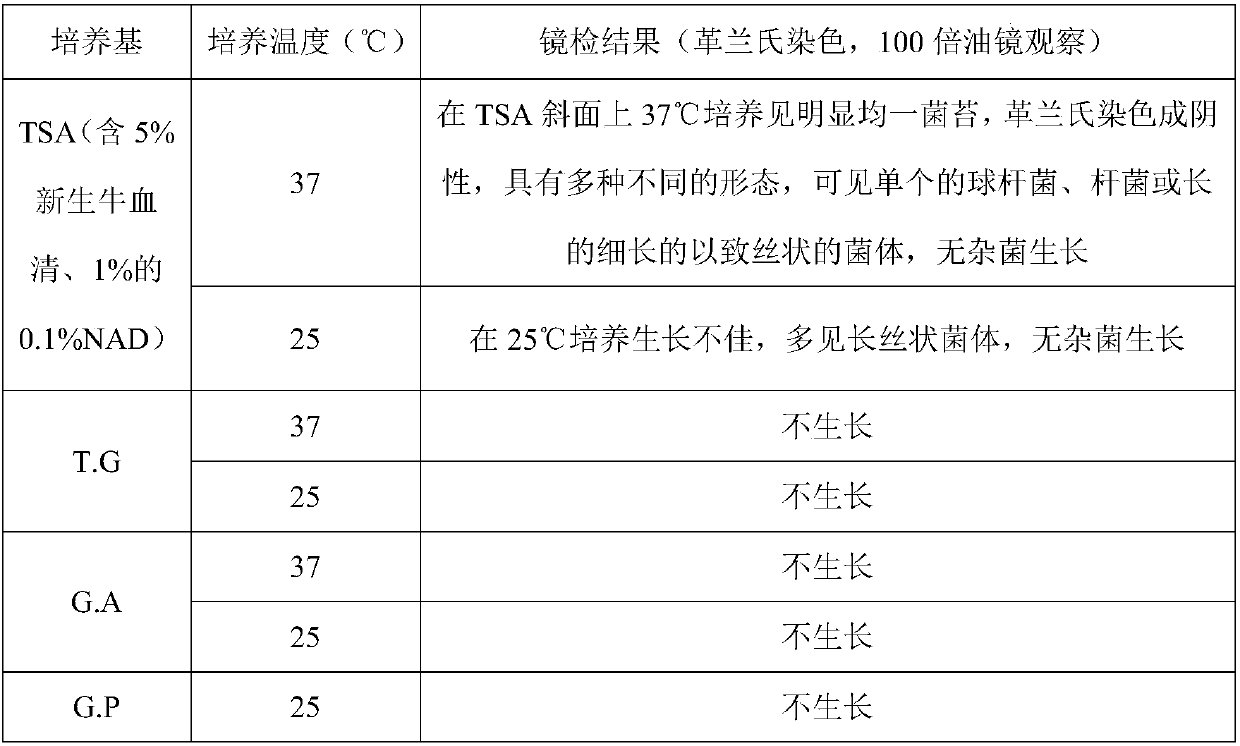
[0028] Isolation and identification of natural attenuated strain of Haemophilus parasuis type 7:
[0029] 1) Strains
[0030] Haemophilus parasuis type 7 natural attenuated strain, isolation number HPS1712, was isolated from the nasal cavity of a healthy piglet in a pig farm in Wuyang County, Henan Province on February 6, 2007, and was isolated and identified by the State Key Laboratory of Agricultural Microbiology, Huazhong Agricultural University and save.
[0031] Haemophilus parasuis type 5 SH0165 strain, type 4 MD0322 strain, and type 13 FX2002 strain were isolated, identified and preserved by the State Key Laboratory of Agricultural Microbiology of Huazhong Agricultural University as the strains for testing.
[0032] 2) Experimental animals
[0033] 16-20g healthy Balb / c mice; 28-35-day-old healthy piglets, purchased from Hubei Wuhan Wannianqing Animal Husbandry Co., Ltd., tested by Haemophilus parasuis antibody ELISA detection kit (self-built method) and the antibody ...
Embodiment 2
[0058] The Haemophilus parasuis type 7 natural attenuated strain HPS1712 that embodiment 1 obtains carries out pathogenicity detection:
[0059] 1) Determination of toxicity to mice
[0060] 130 healthy Balb / c mice weighing 16-20 g were randomly divided into 13 groups, 10 in each group. The Haemophilus parasuis HPS1712 strain and the virulent strain SH0165 were divided into 6 gradients according to different doses, each strain was injected into 6 groups of mice, and the bacterial solution was injected into the chest cavity with 0.5ml / mouse, and the number of viable bacteria was 7.7 ×10 7 CFU, 9.4×10 8 CFU, 1.3×10 9 CFU, 1.7×10 10 CFU, 2.1×10 11 CFU, 1.7×10 12 CFU, another group was injected with 0.5ml PBS as a control, observed for 14 days after the injection, and counted the mortality of mice in each group, the results are shown in Table 4 below.
[0061] Table 4 Haemophilus parasuis type 7 HPS1712 strain and virulent strain SH0165 strain are to the infection test resu...
Embodiment 3
[0069] Detection of immune efficacy of Haemophilus parasuis type 7 natural attenuated strain HPS1712:
[0070] 1) Preparation of the vaccine:
[0071] S1. Cell culture:
[0072] Inoculate the bacterial powder of Haemophilus parasuis HPS1712 strain on the TSA / V / S solid medium, and incubate at 37°C for 24 hours; pick a single colony and re-inoculate the TSA / V / S solid medium for 3 to 5 generations. Incubate at 37°C for 12-16 hours, then pick a single colony on the plate and inoculate it in TSB / V / S liquid medium for 12 hours of activation. After passing the pure inspection, transfer the single colony according to the volume ratio of the bacterial solution to the medium 1:100 To a large bottle of TSB / V / S liquid medium, 37 ° C, 170rpm shaker shaker expansion culture for 12h, the TSA / V / S and TSB / V / S medium is a medium containing 5% bovine serum, 0.2% NAD ;
[0073] S2. Cell collection:
[0074] Transfer the bacterial solution cultured in the liquid in step S1 into a 50ml sterile ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com