Preparation method of triple inactivated vaccine for pigs
An inactivated vaccine and inactivated technology, which is applied in the field of preparation of triple inactivated vaccines for pigs, can solve the problems of lack of vaccine preparation methods for Actinobacillus, reduce the risk of adverse product reactions, reduce immune side effects, and ensure immunity original effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
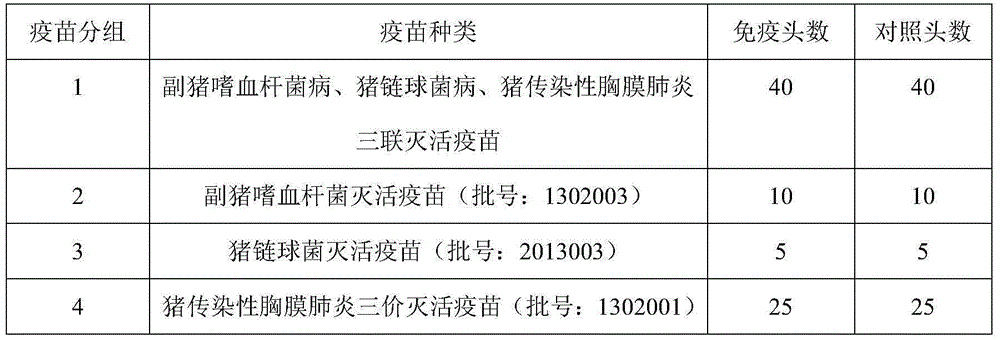
[0033] 1. Vaccine preparation
[0034] A preparation method of Haemophilus parasuis, Streptococcus suis, and porcine infectious pleuropneumonia triple inactivated vaccine, the steps are as follows:
[0035] 1 Production strain preparation
[0036] 1.1 First-class seed propagation
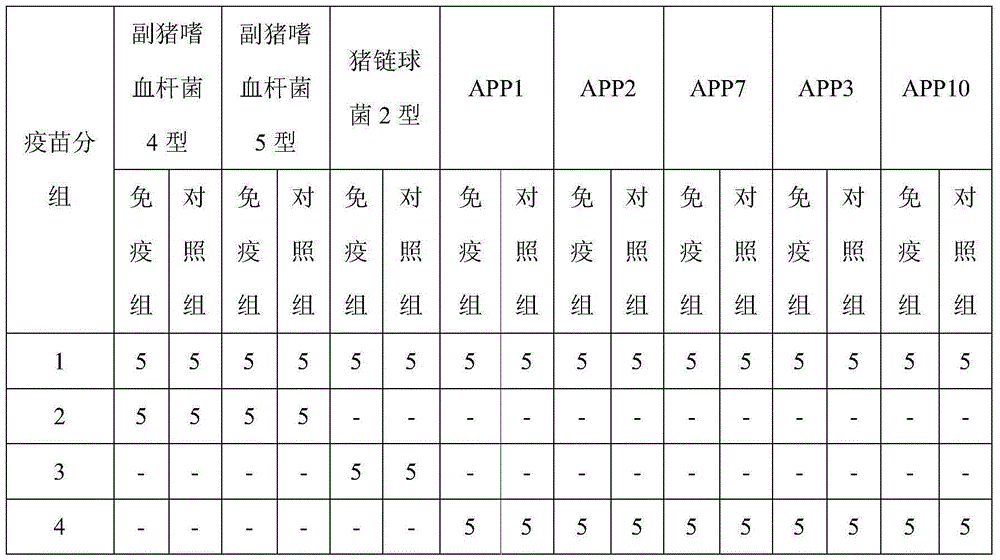
[0037] Haemophilus parasuis type 4, Haemophilus parasuis type 5, Streptococcus suis type 2, Actinobacillus pleuropneumoniae type 3, Actinobacillus pleuropneumoniae type 7, Porcine infectious pleuropneumoniae Freeze-dried strains of Actinobacillus type 10 were streaked and inoculated on TSA / NAD plates, cultured at 37°C for 18-24 hours, picked a single colony and inoculated in TSB / NAD liquid medium, and cultured at 37°C for 12-16 hours , is the primary seed.
[0038] 1.2 Secondary seed propagation
[0039] The prepared Haemophilus parasuis type 4, Haemophilus parasuis type 5, Streptococcus suis type 2, Actinobacillus pleuropneumoniae type 3, Actinobacillus pleuropneumoniae type 7, porcine infectio...
Embodiment 2
[0089] A preparation method for pig triple inactivated vaccine, comprising the following steps:
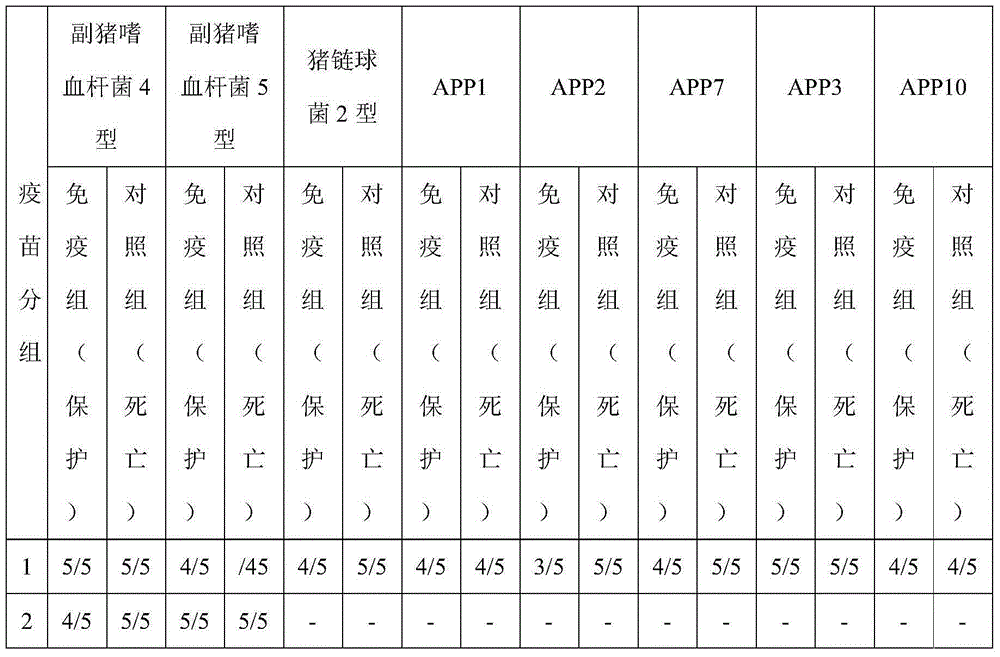
[0090] 1) Take the culture solution of Actinobacillus pleuropneumoniae type 3, the culture solution of Actinobacillus pleuropneumoniae type 7, and the culture solution of Actinobacillus pleuropneumoniae type 10 respectively, and separate the solid and liquid to collect the supernatant ;
[0091] 2) Concentrate the supernatants described in step 1) through ultrafiltration tubes, respectively add saturated ammonium sulfate to obtain precipitates, inactivate them respectively, concentrate them respectively, and adjust the toxin concentration to 200ug / mL respectively, thereby obtaining Apx Ⅰ antigen solutions respectively , Apx Ⅱ antigen solution and Apx Ⅲ antigen solution;
[0092] 3) Take Haemophilus parasuis type 4 culture solution, Haemophilus parasuis type 5 culture solution, and Streptococcus suis type 2 culture solution respectively, inactivate and concentrate respectively, an...
Embodiment 3
[0104] A preparation method for pig triple inactivated vaccine, comprising the following steps:
[0105] 1) Take the culture solution of Actinobacillus pleuropneumoniae type 3, the culture solution of Actinobacillus pleuropneumoniae type 7, and the culture solution of Actinobacillus pleuropneumoniae type 10 respectively, and separate the solid and liquid to collect the supernatant ;
[0106] 2) Concentrate the supernatants described in step 1) through ultrafiltration tubes, respectively add saturated ammonium sulfate to obtain precipitates, inactivate and concentrate respectively, and then adjust the toxin concentration to 400ug / mL, thereby obtaining Apx Ⅰ antigen solutions respectively , Apx Ⅱ antigen solution and Apx Ⅲ antigen solution;
[0107] 3) Take Haemophilus parasuis type 4 culture solution, Haemophilus parasuis type 5 culture solution, and Streptococcus suis type 2 culture solution respectively, inactivate and concentrate respectively, and then adjust the cell conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com