Novel haemophilus parasuis disease trivalent inactivated vaccine and preparation method thereof
A technology for Haemophilus suis disease and Haemophilus suis, applied in antibacterial drugs, pharmaceutical formulations, bacterial antigen components, etc., can solve the problem of difficult to achieve immune effect, difficulty in prevention and treatment, and interactive immunity of Haemophilus parasuis serogroups. It can effectively treat and prevent Haemophilus parasuis related diseases, achieve good immune effect, and solve the problems of preparation difficulties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
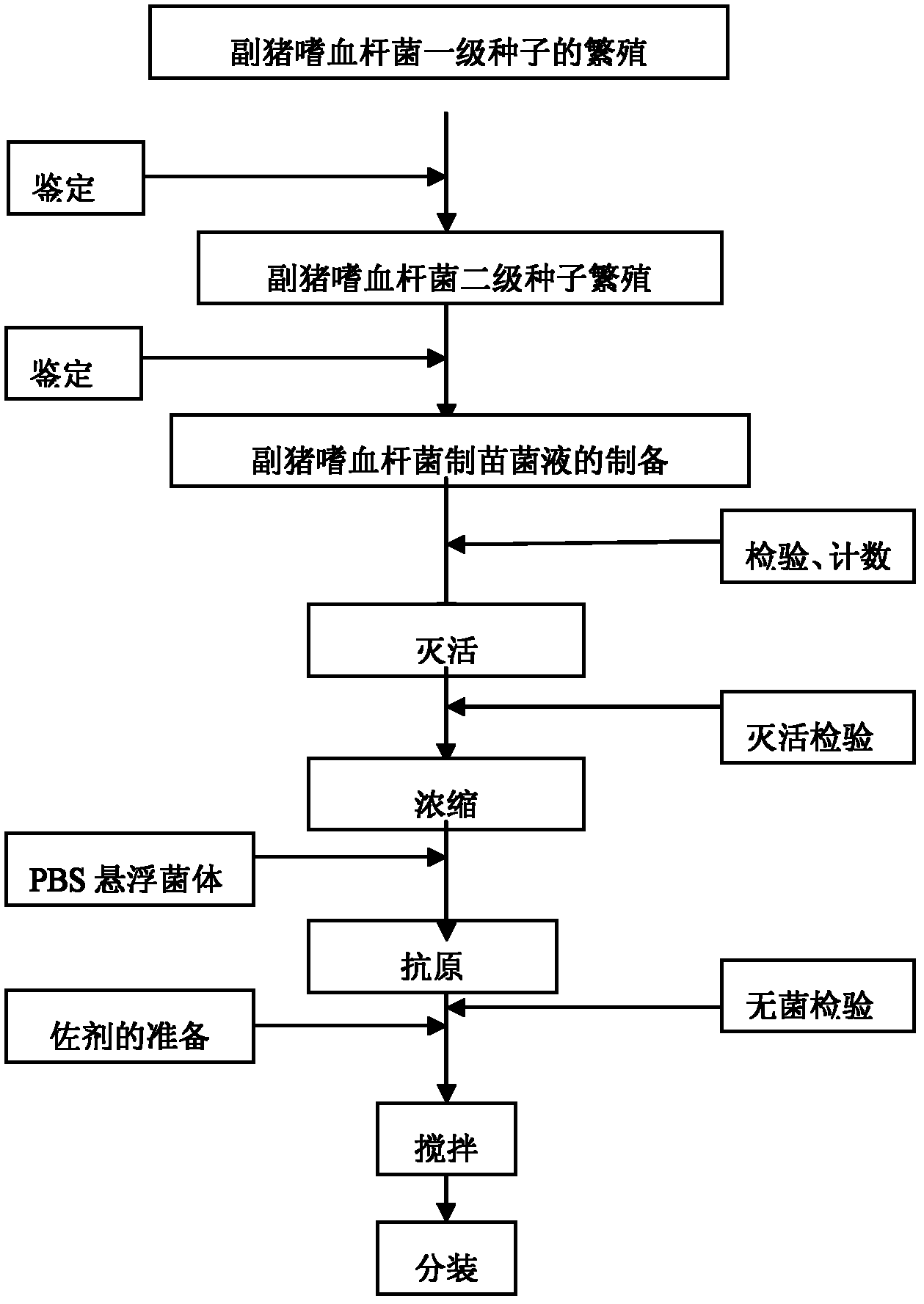
[0039] The preparation method of novel Haemophilus parasuis inactivated vaccine comprises the following steps:
[0040] a. Breeding and culturing the H. parasuis serum type 4 JS strain, type 5 ZJ strain, and type 12 HeB strain bacteria respectively to obtain type 4 JS strain bacterial fluid, type 5 ZJ strain bacterial fluid, and type 12 HeB strain bacterial fluid ;
[0041] b. Add 0.3% formaldehyde solution in total to the Type 4 JS strain bacterial liquid, Type 5 ZJ strain bacterial liquid, and Type 12 HeB strain bacterial liquid obtained in step a, respectively, place it at 37°C for inactivation, and stir for 3~ 5 times, concentrate the bacterial solution according to the counting result;
[0042] c. Mix the above-mentioned inactivated and concentrated type 4 JS strain bacterial liquid, type 5 ZJ strain bacterial liquid, and type 12 HeB strain bacterial liquid in equal proportions, and add 0.01% thimerosal in the total amount of the vaccine, and then carry out with differen...
Embodiment 1
[0047] Preparation and efficacy experiment of embodiment 1 Haemophilus parasuis trivalent inactivated vaccine
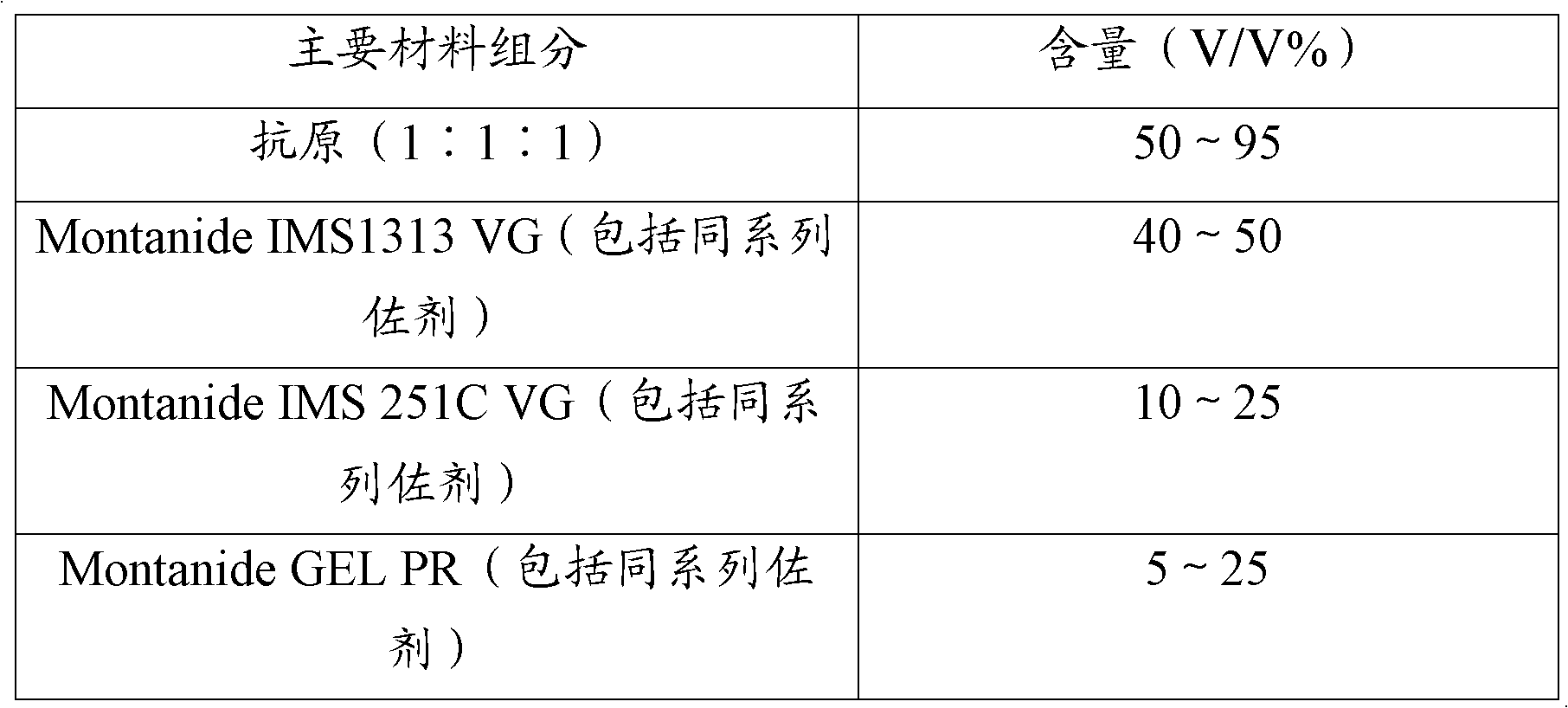
[0048] The trivalent inactivated vaccine for Haemophilus parasuis disease is prepared from the strains of Haemophilus parasuis serum type 4, 5, and 12 that have been inactivated, concentrated, and passed the safety inspection. Antigens prepared from type 4, type 5, and type 12 strains were mixed with Montanide IMS1313 VG (including the same series of adjuvants), Montanide IMS 251C VG (including the same series of adjuvants), Montanide ISA15A VG (including the same series of adjuvants), Montanide GEL PR (including the same series of adjuvants), white oil adjuvant, and aluminum hydroxide gel adjuvant are mixed to prepare the vaccine, wherein the concentration ratio of the three antigens is equal.
[0049] 1. Strains
[0050] a. Haemophilus parasuis serotype 4 JS strain, isolated and identified by Pulaike Bioengineering Co., Ltd., has been preserved in the China Center...
Embodiment 2
[0096] Example 2: Screening of the best adjuvant for Haemophilus parasuis vaccine
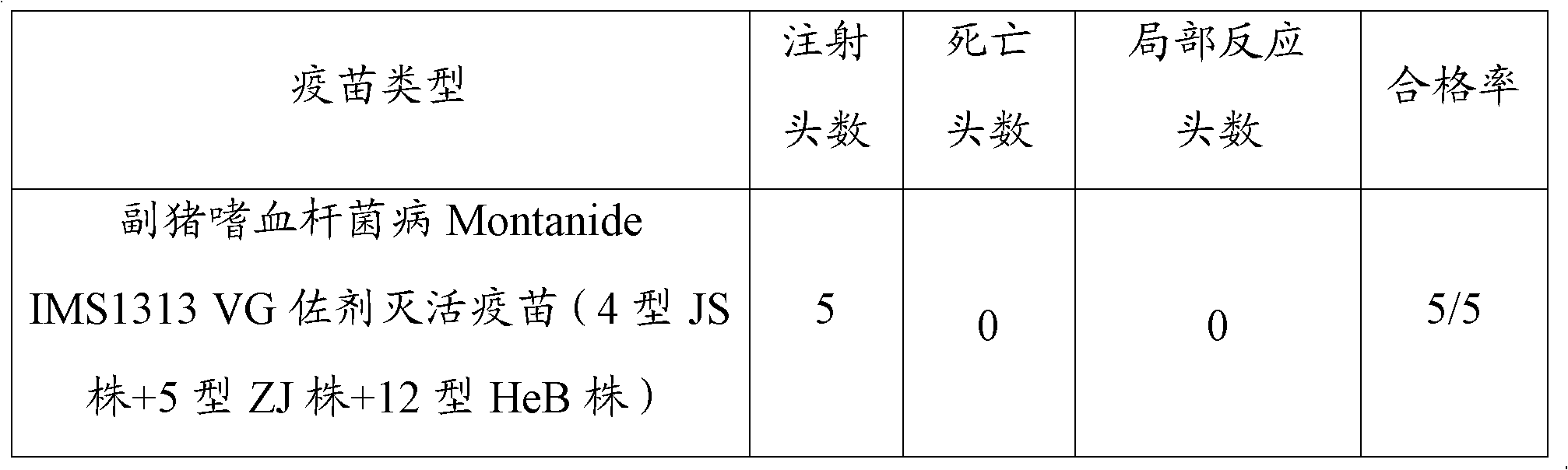
[0097] (1) Safety: The vaccine prepared in Example 1 was injected intramuscularly into 5 healthy susceptible pigs, each with 4 mL, and there was no local reaction within 14 days and all were healthy and alive. The test results are as follows in Table 1:
[0098] Table 1 Results of vaccine trials
[0099]
[0100]
[0101] (2) Efficacy test:
[0102] Experiments were carried out in the experimental animal room of Pulaike Bioengineering Co., Ltd. Get the vaccine prepared in Example 1, each group of vaccines uses 15 healthy susceptible pigs (raised and provided by Pulaike Bioengineering Co., Ltd. Experimental Animal Room) for each group of vaccines, and each intramuscularly injects 2 mL, containing 1 dosage After 28 days, the immune test pigs injected with each vaccine were randomly divided into three groups, and were challenged with the serotype 4, 5 and 12 strains of Haemophilus parasui...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com